An updated narrative review of treatment for limbal epithelial stem cell deficiency
阅读量:1031
DOI: 10.21037/aes-22-51
发布日期:2023-09-08
作者:
Choul Yong Park
展开更多 '%20fill='white'%20fill-opacity='0.01'/%3e%3cmask%20id='mask0_3477_29692'%20style='mask-type:luminance'%20maskUnits='userSpaceOnUse'%20x='0'%20y='0'%20width='16'%20height='16'%3e%3crect%20id='&%23232;&%23146;&%23153;&%23231;&%23137;&%23136;_2'%20x='16'%20width='16'%20height='16'%20transform='rotate(90%2016%200)'%20fill='white'/%3e%3c/mask%3e%3cg%20mask='url(%23mask0_3477_29692)'%3e%3cpath%20id='&%23232;&%23183;&%23175;&%23229;&%23190;&%23132;'%20d='M14%205L8%2011L2%205'%20stroke='%23333333'%20stroke-width='1.5'%20stroke-linecap='round'%20stroke-linejoin='round'/%3e%3c/g%3e%3c/g%3e%3c/svg%3e)
关键词
Limbus
stem cell
cornea
deficiency
transplantation
摘要
Background and Objective: Nearly 30 years have passed since limbal stem cell deficiency (LSCD) was first identified by pioneers and given clinical attention. LSCD remains a difficult disease to treat. It can potentially lead to blinding. At present, understanding of limbal stem cells (LSCs) has deepened and various treatment options for LSCD have been devised. The objective of this review is to summarize basic knowledge of LSCD and current treatment strategies.
Methods: PubMed search was performed to find studies published in English on LSCs and LSCD including original reports and reviews. Literatures published from 1989 to 2022 were reviewed.
Key Content and Findings: LSCs are enigmatic stem cells for which no specific marker has been discovered yet. Although LSCD is not difficult to diagnose, it is still challenging to treat. An important advancement in the treatment of LSCD is the provision of guidelines for selecting systematic surgical treatment according to the patient’s condition. It is also encouraging that stem cell technologies are being actively investigated for their potential usefulness in the treatment of LSCD.
Key Content and Findings: LSCs are enigmatic stem cells for which no specific marker has been discovered yet. Although LSCD is not difficult to diagnose, it is still challenging to treat. An important advancement in the treatment of LSCD is the provision of guidelines for selecting systematic surgical treatment according to the patient’s condition. It is also encouraging that stem cell technologies are being actively investigated for their potential usefulness in the treatment of LSCD.
Conclusions: Although various treatment options for LSCD have been developed, it should be kept in mind that the best chance of treatment for LSCD is in the early stage of the disease. Every effort should be made to preserve as many LSCs as possible in the early treatment of LSCD.
全文
Introduction
Background
Limbal stem cell deficiency (LSCD) is a rare disease. Its treatment is often difficult. LSCD is a condition in which normal corneal epithelization is not maintained because of the loss of limbal stem cells (LSCs) (1,2). Diseased corneal surface is covered by opaque conjunctiva with neovascularization. In severe conditions involving the visual axis, visual rehabilitation should be achieved using stepwise surgical approaches including multiple surgeries such as LSC transplantation and corneal transplantation.
Rationale and knowledge gap
Since the pioneering works by Kenyon and Tseng who first proposed the role of LSCs in corneal epithelial homeostasis in 1989, many basic and clinical studies have been reported in this field (1). Various cell markers for LSCs have been found and innovative surgical treatment modalities have been developed (3).Recently, advanced stem cell technology has been incorporated into the development of a novel cell therapy to replace LSCs.
Objective
The aim of this review was to summarize basic knowledge of LSCD as clinicians and a general concept of its diagnosis and treatment. This article is presented in accordance with the Narrative Review reporting checklist (available at https://aes.
amegroups.com/article/view/10.21037/aes-22-51/rc).
Methods
A PubMed literature review was performed to search for relevant articles on LSCs and LSCD published in English from May 1989 to May 2022. The search strategy is presented in Table 1.
Table 1 Search strategy summary

Discussion
LSCs
It has been known for a long time that stem cells for regenerating corneal epithelium exist. The exact location of these stem cells was found to be the limbus about 30 years ago. Using radio-labeling technique, Cotsarelis et al. (4) have verified that there are label-retaining, slow-cycling, and stem like cells at the limbus. These cells progressively lost radio-labeling as they migrated toward the central cornea.
LSCs reside in the limbal stem cell niche, a unique environment at the junction between the corneal epithelium and the conjunctival epithelium. The limbal stem cell niche has the classical architecture of the palisade of Vogt, limbal stem cell crypt, and limbal crypt or focal stromal projection near the palisade of Vogt (5). These structures are more prevalent in the superior and inferior limbus. The niche provides protection from ultraviolet light and supplies LSCs with blood vessels and various signaling molecules.Recently, there were some evidences in animals and human to support the existence of LSCs even at the central cornea(6-8). However, it is still an established theory that most LSCs reside in the limbal area.
Molecular markers of LSCs are important for isolating and harvesting LSCs and culturing them for transplantation.For a long time, many researchers have tried to find molecular markers to characterize LSCs. However, there are no established markers for LSCs yet. At present, cytokeratins (such as cytokeratin K15), ΔNp63α, C/EBPσ, Bmi1, ABCG2,and Notch-1 are well known candidate markers for LSCs (9).
Limbal stem cell niche
The LSC niche is located in the Palisades of Vogt that are radially oriented, usually pigmented, and more prominent in superior and inferior limbi. Unlike the cornea, basement membrane at Palisades of Vogt is undulating with papillae, crypts, or stromal projections and fenestrated. This unique structure can shelter LSCs from physical shearing stress and provide a larger surface area to accommodate more LSCs within the confined area. Pigments of surrounding melanocyte can protect LSCs from ultraviolet damage (10).Limbal stroma underlying the Palisades of Vogt is heavily innervated and vascularized.
The interaction of stem cell with the surrounding environment such as vessel, neurons, and extracellular matrix is important in the homeostasis or activation of stem cells. How LSCs divide and maintain homeostasis has not been clarified yet. However, many studies have speculated that the unique three-dimensional structure of the limbus might play a critical role. Characteristically, the basement membrane at the Palisades of Vogt expresses laminin α2β2 chains, whereas the corneal basement membrane does not.In addition, α1, α2, and α5 chains of type IV collagen are present in the limbal basement membrane, while α3 and α5 chains are present in the central cornea. These properties may assist in sequestering and modulating concentrations of growth factors and cytokines for efficient and precise targeting to LSCs (11).
Etiology of LSCD
LSCD can be acquired or congenital. In most cases, it is acquired (12). The most common etiologies of LSCD are chemical/thermal burns, allergic conjunctivitis, Stevens Johnson syndrome, and mucous membrane pemphigoid,whereas the most common etiology of congenital LSCD is aniridia (2). Common etiologies of LSCD are described in Table 2.
Table 2 Etiology of limbal epithelial stem cell deficiency

Symptoms and signs of LSCD
In its early stages, LSCD is usually asymptomatic. When LSCD progresses, various symptoms can occur, including blurring, ocular discomfort, photophobia, tearing, conjunctiva injection, and pain (3). Patients usually experience these symptoms months to years before diagnosis.
Typical early sign of LSCD is extension of the conjunctival epithelium (slight loss of transparency of epithelium) with fine vessels crossing the anatomic limbus.This is more common in the superior limbus. Punctate epithelial staining, tear film dysfunction, and conjunctival injection can occur when the disease progresses. At the late stage, corneal vascularization (superficial and deep) and fibrovascular pannus can develop and lead to visual loss.
Diagnosis of LSCD
The diagnosis of LSCD is primarily based on slit-lamp findings. An irregular corneal epithelium and conjunctivalization with neovascularization of the involved area suggest LSCD. Close observation of the corneal limbus may reveal disappearance of the palisade of Vogt.Fluorescein staining highlights the irregular, whorl-like pattern of the corneal epithelium (5). Sometimes, the demarcation line between the cornea epithelium and the invading conjunctival epithelium can be observed (12).
Persistent epithelial defect is another important sign of LSCD. When the disease progresses further, corneal melting and perforation can occur.
Various objective diagnostic tools for LSCD are available. Impression cytology can reveal the presence of goblet cells in the conjunctivalized area (12). However, the absence of goblet cells does not necessarily imply intact LSCs. Additional immunocytochemistry is useful to find cells expressing conjunctival epithelial markers (such as cytokeratins 7, 13, and 19) or goblet cell markers such as mucin 5AC (13,14). Cytokeratin 12 is a corneal epithelial cell marker (15). Goblet cells and decreased density of the sub-basal nerve plexus can be detected in patients with LSCD using non-invasive in vivo confocal microscopy (16). LSCD can induce subtle changes in cornea epithelial layers, such as thinning of the epithelial layer or increased reflectivity of the epithelium. These changes can be imaged using anterior segment optical coherence tomography (OCT) (17). OCT angiography can also detect limbal vascular changes in patients with LSCD (18).
Medical treatment of LSCD
Except for sudden trauma such as chemical burn, diseases causing LSCD can slowly deplete LSCs. The damage is further promoted by uncontrolled inflammation, unstable tear film, and eyelid abnormalities. Therefore, treatment is needed to minimize LSC damage at an early stage of the disease to prevent further compromise of residual LSCs.Correction of the eyelid and tear film abnormalities with effective control of ocular surface inflammation may prevent the progression of LSCD to symptomatic stages.
Growth factor therapy is also effective in protecting the remaining LSCs in the limbal niche (3). Autologous serum, platelet-rich plasma, and amniotic membrane extract eyedrops contain various growth factors that can revitalize the limbal niche have the potential to reverse LSCD at an early stage (19). However, these treatments have limited efficacy for LSCD at its advanced stages.
Surgical procedures
Mild degree of partial LSCD can be treated by simply removing abnormal epithelium and allowing the denuded cornea to be resurfaced with cells from the remaining intact LSCs. However, if LSCD has progressed with invasion of the visual axis, advanced surgical treatment is required. The goal of surgery is to transfer a sufficient amount of LSCs or corneal epithelial-like cells to the diseased ocular surface to promote and maintain corneal epithelialization. Although many types of limbal epithelial cell transplantation technique have been developed, none of them is universally successful.
Among various surgical methods introduced, the choice of surgical methods should be based on a thorough consultation between the patient and the surgeon. Depending on the surgical method, the patient may be at risk of damage to the healthy contralateral eye or long-term systemic immunosuppression if an allograft is used. Harvesting LSCs from the contralateral eye (e.g., conjunctival limbal autograft, CLAU) can induce iatrogenic LSCD in the donor eye (20). To avoid the risk
of iatrogenic LSCD or bilateral advanced LSCD where enough autologous LSCs cannot be harvested, cell culture methods such as autologous cultivated limbal epithelial transplantation (CLET) can be used (21). Alternatively, simple limbal epithelial transplantation (SLET) recently introduced that requires only the use of 2×2 mm-sized small limbal block from the healthy donor eye can be used to minimize the risk of iatrogenic LSCD (22).
SLET deserves further discussion. Other surgical methods developed so far can transplant the harvested graft onto the limbus, the primary area of disease. However, in SLET, harvested limbal graft is cut into 6–10 pieces.These pieces are placed in the mid-periphery of cornea in a concentric pattern using fibrin glue (23). In some cases, human amniotic membrane graft is applied first followed by SLET. The hypothesis supporting SLET is that LSCs in each piece of graft can proliferate and form epithelial clump or island, which can then merge with each other to reconstruct corneal epithelial layer. LSCs may remain around the grafted area or slowly migrate toward the limbus (24). In a long-term (median: 1.5 years, range:1 to 4 years) outcome analysis of 125 cases of SLET in unilateral ocular surface burn, 76% of eyes maintained successful outcomes and 67% of successful cases achieved 20/60 or better vision (25). In addition, analysis of 10 cases
underwent penetrating keratoplasty for further visual gain revealed that basal cells expressing limbal stem cell markers [ΔNp63α(+)/ABCG2(+)] were observed in the trephined cornea after undergoing SLET (25).
In general, autografts have high success rates and better long-term prognosis. However, when LSCs cannot be obtained from the patient because of advanced bilateral LSCD, the use of allogeneic cells may be considered if the patient can tolerate systemic immunosuppression. The major limitation of an allograft is the risk of graft rejection because of the high vascular nature at the limbus. Allogeneic limbal stem cells can be used for conjunctival limbal
allograft (CLAL), allo-SLET, allo-CLET, and keratolimbal allograft (KLAL).
If patients cannot tolerate long-term immunosuppressive treatment owing to their general conditions, surgical methods using cells from other body parts of the patient,even those without LSCs, should be considered. Cultivated oral mucosal epithelial transplantation (COMET) can be used to harvest autologous oral mucosal cells, to culture cells in vitro on carriers such as the amniotic membrane,or as a cell sheet for transplanting on the diseased ocular surface (26,27).
Cabral et al. (28) have analyzed clinical outcomes of COMET studies published from 2004 to 2019. Among 243 eyes from 24 studies, 70.8% of eyes achieved a stable ocular surface. Visual acuity improvement was observed in 63.5% of eyes. It is known that the presence of pre-operative epithelial defect and poor tear production can increase the failure rate of COMET. Therefore, these abnormalities should be corrected prior to surgery for better outcome. Although several studies reported that the oral mucosal cells grafted with COMET could transdifferentiate into corneal epithelial phenotype expressing cytokeratin such K12 (29,30) without expressing MUC5AC, it is still difficult to definitively determine whether cultivated oral mucosal epithelial cells have transdifferentiated to the corneal lineage or whether the presence of corneal epithelial cells indicates expansion and migration of the remaining corneal cells.
With advances in stem cell technology, other stem cell populations can be transdifferentiated to mimic corneal epithelial cells. Although this is still at the laboratory level, it is a promising option for future treatment of LSCD.
Figure 1 shows an example of how to select an appropriate surgical method according to various patient conditions. The flow chart was modified from suggestions of two recent publications (3,20).

Figure 1 Flowchart of current surgical therapeutic options for LSCD. LSCD, limbal stem cell deficiency; AMT, amniotic membrane
transplantation; CLAU, conjunctival limbal autograft; SLET, simple limbal epithelial transplantation; CLET, cultivated limbal epithelial transplantation; KLAL, keratolimbal allograft; CLAL, conjunctival limbal allograft; COMET, cultivated oral mucosal epithelial transplantation.
Alternative cells to replace LSCs
With the advancement of stem cell technology, various attempts to use alternative cell populations to replace allogeneic cells are underway. These alternative cell populations can be utilized as limbal stem cell substitutes for ocular surface reconstruction. Pluripotent stem cells, oral mucosa cells, dental pulp stem cells, and hair follicle cells can be cultured on the carrier substrate and transplanted to the eye with severe LSCD. The implanted cell population can transdifferentiate into more corneal epithelial-like cells and maintain ocular surface integrity and transparency. Although the usefulness of these cells is still being verified in the preclinical stages, corneal limbus reconstruction using these cells is possible in the future (Table 3).
Table 3 Alternative cell populations for treating LSCD
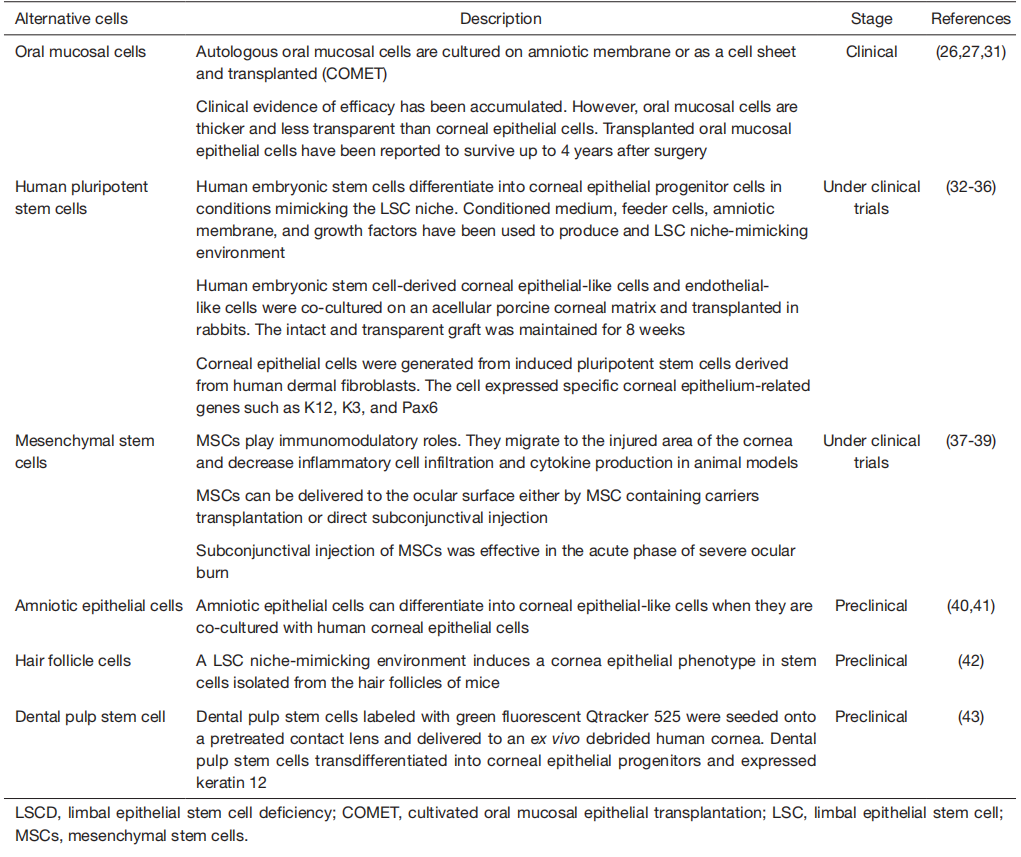
Among alternative cells, mesenchymal stem cells (MSCs) deserve further explanation. In general, MSCs can be obtained from bone marrow or adipose tissues of patients.Transplanted MSCs can differentiate into corneal epithelial cells (44). Although a sufficient number of MSCs do not differentiate into corneal epithelial-like cells, transplanted MSCs are known to have anti-angiogenic and anti-inflammatory properties (45). In addition, the secretomes of cultured limbal MSCs can promote corneal epithelial regeneration (46). MSCs found in the normal limbal stem cell niche are believed to be able to crosstalk with LSCs and control LSC proliferation and differentiation (3,47). Therefore, an exogenous supply of MSCs is expected to secrete trophic and growth factors to stimulate residual LSCs, to control inflammation preventing further damage to LSCs, and finally to improve the limbal niche microenvironment. The non-immunogenic nature of MSCs allows them to be used allogeneically without eliciting immunosuppression of the recipient. Several routes of MSCs application in LSCD are possible. Intravenous injection of MSC in acute phase of cornea damage can alleviate ocular signs such as opacity and neovascularization in animal models (48,49). Various carriers including amniotic membrane, contact lens, and biocompatible polymers can be used to deliver MSCs on the ocular surface (50-52). However, the actual number of transplanted MSCs
in this method is known to be small compared to injection methods. Subconjunctival injection of MSCs has also been proven to be effective in LSCD (37). MSCs can migrate to the inflammatory area from the subconjunctival space and modulate inflammation and tissue damage. There is still debate whether injected MSCs can change to LSCs.
Reprogrammed cells called induced pluripotent stem cells (iPSCs) are also promising stem cell sources to replace LSCs (32,53,54). iPSCs can be induced by culture in a cocktail of signaling factors. LSCs induced from iPSCs may allow personalized therapy according to the patient’s need without the risk of allogeneic rejection. Recently, iPSC derived corneal epithelial sheet have been transplanted to a patient suffering bilateral LSCD in Japan (55). Although clinical results have not yet been reported, this clinical trial shows clear advances in LSCD treatment.
Keratoprosthesis for advanced bilateral LSCD
When bilateral LSCD is combined with severe dry eye,fornix adhesion, lid abnormality, or previous failures of LSC transplantation, the success of LSC transplantation is limited and the use of keratoprostheses can be considered (12,56). Keratoprostheses do not require long-term systemic immunosuppression. Early visual rehabilitation is another advantage of keratoprostheses. However, risks of retinal detachment, endophthalmitis, glaucoma, and implant extrusion are serious limitations (56). In keratoprosthesis surgery, epithelialization of the corneal surface by the corneal epithelium is abandoned. Instead, an implant is inserted to replace the optically transparent cornea,allowing light to reach the retina. After keratoprosthesis surgery, the ocular surface is totally covered with the conjunctival epithelium except for a small optical opening in the implant. The most popular keratoprostheses are Boston KPro, AutoKPro, LV Pradad KPro, and modified osteo-
odonto-keratoprosthesis (MOOKP). Among these, Boston KPro type 1 is the most commonly used keratoprosthesis. It has an optical cylinder with a skirt of donor tissue. Good outcomes of visual rehabilitation have been reported with Boston KPro type. However, retroprosthetic membrane and corneal melting are common complications (57,58). In a retrospective analysis of 23 eyes receiving Boston type 1 keratoprosthesis, postoperative corrective visual acuity of 20/50 or better was obtained in 67% of eyes at a 3-year follow up and persistent corneal epithelial defect and corneal necrosis were observed in 56.5% and 30% of eyes, respectively (59).
Limitations of this review
This review described basic knowledge of LSC and various treatment options for LSCD. Since the purpose of this review was to convey knowledge that a general ophthalmologist or ophthalmology resident could understand, contents that were too specialized or experimental had to be excluded. In addition, only studies published in English and full-length articles retrieved from PubMed search were included in this review. This approach has a limitation in that it does not include latest research studies presented as abstracts in recent academic conferences.
Conclusions
Our understanding of LSCs and LSCD has deepened over the past years. However, much details of LSCs or LSCs niche remains unknown as interesting future topics of research. Despite the enigmatic identity of LSCs, various treatments have been devised and proven effective in clinical practice. However, it should be kept in mind that the best chance of treating LSCD is in the early stages of the disease. In some cases, initial treatment is difficult, especially for hereditary cases,cases with frequent relapses, and cases with intractable underlying etiologies. Once LSCD has progressed,treatment becomes challenging. Nevertheless, in the early treatment of LSCD, every effort should be made to preserve as many LSCs as possible.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joann Kang and Roy S. Chuck) for the series “Ocular Surface Reconstruction/Transplantation”published in Annals of Eye Science. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://aes.amegroups.com/article/view/10.21037/aes-22-51/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aes.amegroups.com/article/view/10.21037/aes-22-51/coif). The series “Ocular Surface Reconstruction/Transplantation” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license).See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
基金
暂无基金信息
参考文献
1、Kenyon KR, Tseng SC. Limbal autograft transplantation
for ocular surface disorders. Ophthalmology 1989;96:709-
22; discussion 722-3.
2、Vazirani J, Nair D, Shanbhag S, et al. Limbal Stem Cell
Deficiency-Demography and Underlying Causes. Am J
Ophthalmol 2018;188:99-103.
3、Elhusseiny AM, Soleimani M, Eleiwa TK, et al. Current
and Emerging Therapies for Limbal Stem Cell Deficiency.
Stem Cells Transl Med 2022;11:259-68.
4、Cotsarelis G, Cheng SZ, Dong G, et al. Existence of
slow-cycling limbal epithelial basal cells that can be
preferentially stimulated to proliferate: implications on
epithelial stem cells. Cell 1989;57:201-9.
5、Haagdorens M, Van Acker SI, Van Gerwen V, et al. Limbal
Stem Cell Deficiency: Current Treatment Options and
Emerging Therapies. Stem Cells Int 2016;2016:9798374.
6、Bi YL, Bock F, Zhou Q, et al. Central corneal epithelium
self-healing after ring-shaped glycerin-cryopreserved
lamellar keratoplasty in Terrien marginal degeneration. Int
J Ophthalmol 2013;6:251-2.
7、Majo F, Rochat A, Nicolas M, et al. Oligopotent stem cells
are distributed throughout the mammalian ocular surface.
Nature 2008;456:250-4.
8、Dua HS, Miri A, Alomar T, et al. The role of limbal stem
cells in corneal epithelial maintenance: testing the dogma.
Ophthalmology 2009;116:856-63.
9、Joe AW, Yeung SN. Concise review: identifying limbal
stem cells: classical concepts and new challenges. Stem
Cells Transl Med 2014;3:318-22.
10、Ordonez P, Di Girolamo N. Limbal epithelial stem
cells: role of the niche microenvironment. Stem Cells
2012;30:100-7.
11、Li W, Hayashida Y, Chen YT, et al. Niche regulation
of corneal epithelial stem cells at the limbus. Cell Res
2007;17:26-36.
12、Kate A, Basu S. A Review of the Diagnosis and Treatment
of Limbal Stem Cell Deficiency. Front Med (Lausanne)
2022;9:836009.
13、Barbaro V, Ferrari S, Fasolo A, et al. Evaluation of
ocular surface disorders: a new diagnostic tool based
on impression cytology and confocal laser scanning
microscopy. Br J Ophthalmol 2010;94:926-32.
14、Poli M, Janin H, Justin V, et al. Keratin 13 immunostaining
in corneal impression cytology for the diagnosis of
limbal stem cell deficiency. Invest Ophthalmol Vis Sci
2011;52:9411-5.
15、Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S,
et al. Keratin 13 is a more specific marker of conjunctival
epithelium than keratin 19. Mol Vis 2011;17:1652-61.
16、Nubile M, Lanzini M, Miri A, et al. In vivo confocal
microscopy in diagnosis of limbal stem cell deficiency. Am
J Ophthalmol 2013;155:220-32.
17、Banayan N, Georgeon C, Grieve K, et al. Spectral-domain
Optical Coherence Tomography in Limbal Stem Cell
Deficiency. A Case-Control Study. Am J Ophthalmol
2018;190:179-90.
18、Lee WD, Devarajan K, Chua J, et al. Optical coherence
tomography angiography for the anterior segment. Eye
Vis (Lond) 2019;6:4.
19、Liu L, Hartwig D, Harloff S, et al. Corneal
epitheliotrophic capacity of three different blood-derived
preparations. Invest Ophthalmol Vis Sci 2006;47:2438-44.
20、Fernandez-Buenaga R, Aiello F, Zaher SS, et al. Twenty
years of limbal epithelial therapy: an update on managing limbal stem cell deficiency. BMJ Open Ophthalmol
2018;3:e000164.
21、Holland EJ. Management of Limbal Stem Cell Deficiency:
A Historical Perspective, Past, Present, and Future.
Cornea 2015;34 Suppl 10:S9-15.
22、Sangwan VS, Sharp JAH. Simple limbal epithelial
transplantation. Curr Opin Ophthalmol 2017;28:382-6.
23、Sangwan VS, Basu S, MacNeil S, et al. Simple limbal
epithelial transplantation (SLET): a novel surgical
technique for the treatment of unilateral limbal stem cell
deficiency. Br J Ophthalmol 2012;96:931-4.
24、Shanbhag SS, Patel CN, Goyal R, et al. Simple limbal
epithelial transplantation (SLET): Review of indications,
surgical technique, mechanism, outcomes, limitations, and
impact. Indian J Ophthalmol 2019;67:1265-77.
25、Basu S, Sureka SP, Shanbhag SS, et al. Simple Limbal
Epithelial Transplantation: Long-Term Clinical Outcomes
in 125 Cases of Unilateral Chronic Ocular Surface Burns.
Ophthalmology 2016;123:1000-10.
26、Nakamura T, Inatomi T, Sotozono C, et al.
Transplantation of cultivated autologous oral mucosal
epithelial cells in patients with severe ocular surface
disorders. Br J Ophthalmol 2004;88:1280-4.
27、Nishida K, Yamato M, Hayashida Y, et al. Corneal
reconstruction with tissue-engineered cell sheets composed
of autologous oral mucosal epithelium. N Engl J Med
2004;351:1187-96.
28、Cabral JV, Jackson CJ, Utheim TP, et al. Ex vivo cultivated
oral mucosal epithelial cell transplantation for limbal stem
cell deficiency: a review. Stem Cell Res Ther 2020;11:301.
29、Kim YJ, Lee HJ, Ryu JS, et al. Prospective Clinical
Trial of Corneal Reconstruction With Biomaterial-Free
Cultured Oral Mucosal Epithelial Cell Sheets. Cornea
2018;37:76-83.
30、Kolli S, Ahmad S, Mudhar HS, et al. Successful application
of ex vivo expanded human autologous oral mucosal
epithelium for the treatment of total bilateral limbal stem
cell deficiency. Stem Cells 2014;32:2135-46.
31、Ma DH, Hsueh YJ, Ma KS, et al. Long-term survival of
cultivated oral mucosal epithelial cells in human cornea:
generating cell sheets using an animal product-free culture
protocol. Stem Cell Res Ther 2021;12:524.
32、Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal
epithelial cells from induced pluripotent stem cells
derived from human dermal fibroblast and corneal limbal
epithelium. PLoS One 2012;7:e45435.
33、Zhang C, Du L, Pang K, et al. Differentiation of
human embryonic stem cells into corneal epithelial
progenitor cells under defined conditions. PLoS One
2017;12:e0183303.
34、Ahmad S, Stewart R, Yung S, et al. Differentiation of
human embryonic stem cells into corneal epithelial-like
cells by in vitro replication of the corneal epithelial stem
cell niche. Stem Cells 2007;25:1145-55.
35、Zhang C, Du L, Sun P, et al. Construction of tissue-engineered full-thickness cornea substitute using limbal
epithelial cell-like and corneal endothelial cell-like cells
derived from human embryonic stem cells. Biomaterials
2017;124:180-94.
36、Vattulainen M, Ilmarinen T, Viheriälä T, et al. Corneal
epithelial differentiation of human pluripotent stem cells
generates ABCB5+ and ∆Np63α+ cells with limbal cell
characteristics and high wound healing capacity. Stem Cell
Res Ther 2021;12:609.
37、Galindo S, de la Mata A, López-Paniagua M, et al.
Subconjunctival injection of mesenchymal stem cells for
corneal failure due to limbal stem cell deficiency: state of
the art. Stem Cell Res Ther 2021;12:60.
38、Liang L, Luo X, Zhang J, et al. Safety and feasibility of
subconjunctival injection of mesenchymal stem cells for
acute severe ocular burns: A single-arm study. Ocul Surf
2021;22:103-9.
39、Shukla S, Shanbhag SS, Tavakkoli F, et al. Limbal
Epithelial and Mesenchymal Stem Cell Therapy for
Corneal Regeneration. Curr Eye Res 2020;45:265-77.
40、Yao M, Chen J, Yang XX, et al. Differentiation of human
amniotic epithelial cells into corneal epithelial-like cells in
vitro. Int J Ophthalmol 2013;6:564-72.
41、Zhou Q, Liu XY, Ruan YX, et al. Construction of corneal
epithelium with human amniotic epithelial cells and
repair of limbal deficiency in rabbit models. Hum Cell
2015;28:22-36.
42、Meyer-Blazejewska EA, Call MK, Yamanaka O, et al.
From hair to cornea: toward the therapeutic use of hair
follicle-derived stem cells in the treatment of limbal stem
cell deficiency. Stem Cells 2011;29:57-66.
43、Kushnerev E, Shawcross SG, Sothirachagan S, et al.
Regeneration of Corneal Epithelium With Dental Pulp
Stem Cells Using a Contact Lens Delivery System. Invest
Ophthalmol Vis Sci 2016;57:5192-9.
44、Rohaina CM, Then KY, Ng AM, et al. Reconstruction
of limbal stem cell deficient corneal surface with induced
human bone marrow mesenchymal stem cells on amniotic
membrane. Transl Res 2014;163:200-10.
45、Eslani M, Putra I, Shen X, et al. Corneal Mesenchymal
Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci 2017;58:5507-17.
46、Shibata S, Hayashi R, Okubo T, et al. The secretome
of adipose-derived mesenchymal stem cells attenuates
epithelial-mesenchymal transition in human corneal
epithelium. Regen Ther 2019;11:114-22.
47、Yazdanpanah G, Haq Z, Kang K, et al. Strategies for
reconstructing the limbal stem cell niche. Ocul Surf
2019;17:230-40.
48、Mittal SK, Omoto M, Amouzegar A, et al. Restoration of
Corneal Transparency by Mesenchymal Stem Cells. Stem
Cell Reports 2016;7:583-90.
49、Ye J, Yao K, Kim JC. Mesenchymal stem cell
transplantation in a rabbit corneal alkali burn model:
engraftment and involvement in wound healing. Eye
(Lond) 2006;20:482-90.
50、Calonge M, Pérez I, Galindo S, et al. A proof-of-concept
clinical trial using mesenchymal stem cells for the
treatment of corneal epithelial stem cell deficiency. Transl
Res 2019;206:18-40.
51、Holan V, Trosan P, Cejka C, et al. A Comparative
Study of the Therapeutic Potential of Mesenchymal
Stem Cells and Limbal Epithelial Stem Cells for
Ocular Surface Reconstruction. Stem Cells Transl Med
2015;4:1052-63.
52、Espandar L, Caldwell D, Watson R, et al. Application of
adipose-derived stem cells on scleral contact lens carrier
in an animal model of severe acute alkaline burn. Eye
Contact Lens 2014;40:243-7.
53、Takahashi K, Yamanaka S. Induction of pluripotent stem
cells from mouse embryonic and adult fibroblast cultures
by defined factors. Cell 2006;126:663-76.
54、Yu D, Chen M, Sun X, et al. Differentiation of mouse
induced pluripotent stem cells into corneal epithelial-like
cells. Cell Biol Int 2013;37:87-94.
55、Watanabe S, Hayashi R, Sasamoto Y, et al. Human iPS
cells engender corneal epithelial stem cells with holoclone-forming capabilities. iScience 2021;24:102688.
56、Vazirani J, Mariappan I, Ramamurthy S, et al. Surgical
Management of Bilateral Limbal Stem Cell Deficiency.
Ocul Surf 2016;14:350-64.
57、Wang LQ, Wu TY, Chen XN, et al. Long-term outcomes
of Boston keratoprosthesis type I: the Chinese People's
Liberation Army General Hospital experience. Br J
Ophthalmol 2022;106:781-5.
58、Samarawickrama C, Strouthidis N, Wilkins MR. Boston
keratoprosthesis type 1: outcomes of the first 38 cases
performed at Moorfields Eye Hospital. Eye (Lond)
2018;32:1087-92.
59、Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis
in the management of corneal limbal stem cell deficiency.
Cornea 2011;30:1187-94.
相关文章
Katherine Chen;Mohammad Soleimani;Raghuram Koganti;Kasra Cheraqpour;Samer Habeel;Ali R. Djalilian,Cell-based therapies for limbal stem cell deficiency: a literature reviewJennifer C. W. Hu;Danielle Trief,A narrative review of limbal stem cell deficiency & severe ocular surface diseaseLi Wang;Bowen Wang;Hong Ouyang,Limbal epithelial stem cells in corneal surface reconstruction