Diabetic retinopathy: an inflammatory disease
关键词
摘要
Abstract: Diabetic retinopathy (DR) is a complex multifactorial disease and one of the leading causes of visual impairment worldwide. DR pathogenesis is still not completely understood and, even if studies performed in the past focused on microvascular dysfunction as the main event, growing body of scientific evidence has demonstrated an important role of inflammation and neurodegeneration in the onset and progression of DR. This review summarizes current literature on the role of inflammation in the pathogenesis and progression of DR. In particular, it focuses on clinical inflammatory biomarkers detectable with non-invasive retinal imaging, suggestive of a local inflammatory condition. Current available treatments are applicable only at advanced stages of disease, therefore, there is the need to detect biomarkers of subclinical or early DR that can help in DR management before irreversible damage occurs. A better understanding of inflammatory pathways involved in DR may permit to implement more specific and personalized therapeutic strategies and clinical biomarkers may be a helpful tool in the everyday clinical practice to direct the patient to the most appropriate treatment option.
全文
Introduction
Diabetic retinopathy (DR) is an important complication of both type 1 and type 2 diabetes mellitus (1) and the leading cause of preventable blindness in working age population worldwide (2). DR affects about one-third of diabetic population and 10% has sight-threatening complications such as diabetic macular edema (DME) and proliferative DR (PDR) (3). The International Clinical Diabetic Retinopathy Disease Severity Scale divides DR in five different stages based on clinical signs of vascular impairment detectable on fundus examination: no apparent DR, mild, moderate and severe non proliferative DR (NPDR), and PDR, as defined by the presence of retinal neovascularization or vitreous and/or preretinal hemorrhage (4). DR could remain silent for a long period of time and, by the time symptoms become manifest, disease may have already progressed to advanced stages. Functional changes in eyes of diabetic patients can be detected even before the appearance of initial signs of DR, as demonstrated by means of multifocal electroretinogram (mfERG) and visual evoked potentials (VEPs) (5,6). This suggests that damage in neuroretinal function in DR begins before clinically visible vascular changes occur (7-9), as confirmed by recent studies performed with spectral domain (SD)-optical coherence tomography (OCT) and OCT-angiography (10-18). Therefore, there is a need for evaluation and validation of (possibly) non-invasive biomarkers that can be used for early detection of subclinical signs of DR. Even if many previous studies focused on the role of vascular dysfunction in DR pathogenesis, diabetic microvasculopathy cannot completely explain early neuroretinal damage and thus DR could not simply be considered a pure vasculopathy. Functional alterations were demonstrated in the earliest stages of disease before the development of vascular dysfunction (5,6). Even if the exact mechanisms initiating neuroretinal damage in DR are not fully understood, actual evidence focuses on the role of chronic hyper-glycaemia-induced inflammation as critical contributing factor in DR pathogenesis (19,20) and several studies described the association between high levels of systemic and local inflammatory molecules and the development and progression of DR (21-26).
In this review we will first discuss current literature focused on the role of inflammation in the pathogenesis and progression of DR and then we will describe retinal features that may be used as non-invasive clinical inflammatory biomarkers in the everyday clinical practice that may be useful to choose the most appropriate treatment option.
Molecular basis of inflammation in DR
DR has a complex multifactorial etiology and all retinal cellular elements (vascular, neural and glial elements forming the so-called retinal neurovascular unit) are involved in the pathological process (27), even before the onset of clinical signs of DR (28-33). DR is nowadays considered a chronic low-grade inflammatory disorder involving a cascade of inflammatory mediators and adhesion molecules (19,20,34-36). Subclinical chronic inflammation contributes to diabetes occurrence and to the development of its long-term complications, including DR (37). Knowing inflammatory pathways could be helpful to create strategies to prevent and control diabetes and its complications before they cause irreversible organ damage (37).
The major factor determining retinal dysfunction in DR is represented by chronic hyper-glycaemia (19,20). Hyper-glycaemia causes the elevation of intracellular glucose and, consequently, the activation of four different cellular glucose metabolic pathways: the diacylglycerol (DAG)-protein kinase C (PKC), advanced glycation end products (AGE)/AGE receptors (RAGE), sorbitol and hexosamine pathways (38-40). This leads to cellular damage in terms of micro-vascular dysfunction, neuronal apoptosis, glial reactivity and component deposition (38-40). All these interconnected aspects are linked to upregulation of angiogenic and inflammatory mediators and to altered growth factor signaling, with recruitment and infiltration of macrophages, monocytes and neutrophils and consequent aberrant inflammatory response (28,31,32,41-43).
Endothelial dysfunction associated with inflammation determines increased vascular permeability, alteration of blood flow, oxidative stress and angiogenesis and has been related to increased expression of inflammatory adhesion molecules (ICAM-1, VCAM-1 and E-selectin) in the endothelium (44,45). Elevated levels of these molecules lead to adhesion and accumulation of leukocytes within retinal vessels, one of the very early events that occur in diabetic retina inflammation (within one week in experimental diabetes) (46-48). This results in loss of pericytes, formation of acellular capillaries and consequent break-down of the blood-retinal barrier (28,49-51) that progresses toward increased retinal vascular permeability, development of DME, and neovascularization (PDR) (41).
Retinal glial cells including astrocytes, Müller cells (MCs) and microglia play a central structural role and contribute to maintain homeostasis in the retina. Their activation is considered another critical feature involved in the initiation and amplification of inflammation in diabetic neuroretinal dysfunction (52-54). Microglia cells are thought to be the first responders to hyperglycemic stress undergoing a shift in their phenotype from “surveying microglia” to “activated microglia” (55) and migrating from the inner to the outer retinal layers (56-58). Activated microglia cells start to produce pro-inflammatory mediators such as TNF-alpha, IL-6, MCP-1 and VEGF (52,59) amplifying the inflammatory response that triggers MCs reactive gliosis (60), a process consisting of hypertrophy, cellular proliferation and increase in intermediate filament proteins such as glial fibrillary acidic protein (GFAP) (33). Glial activation occurs early in DR pathogenesis, as demonstrated by the upregulation of GFAP and aquaporin 4 (AQP4) produced by MCs both in the retinas of animal and human models of DM and in vivo in human ocular fluids and specifically in the aqueous humor of patients with DM but no DR or with early signs of DR compared to healthy controls (33,61-64).
Even if VEGF is the most studied factor involved in DR pathogenesis and the main target of available therapeutic strategies, its role cannot explain alone all the events taking place in DR onset and progression. VEGF selective inhibition is not sufficient to stop the inflammatory cascade in DR and anti-VEGF therapies are frequently of transient benefit, especially in DME treatment, needing repeated injections over time (65,66) and suggesting the involvement of other molecular pathways. It has been demonstrated that a wide range of systemic and local inflammatory biomarkers are involved and act together in DR: vascular adhesion molecules (VCAM-1, ICAM-1, E-selectin, sVAP), pro-inflammatory cytokines (TNF-alpha, IL-1alpha, 1beta, 6, 8, HMGB1), anti-inflammatory cytokines (IL-10), pro-inflammatory/angiogenic chemokines (CP-1, MIF, SDF-1, fractalkine), anti-inflammatory/antiangiogenic chemokines (IP10, MIG), transcription factors (HIF-1, NF-κB), pro-inflammatory/angiogenic growth factors (VEGF, PGF, IGF1, CTGF, stem cell factor), anti-inflammatory/antiangiogenic growth factors (PEDF), anti-inflammatory/proangiogenic growth factors (EPO), and innate immune response cells (retinal endothelial cells with toll-like receptors) (67). In addition, there is some evidence suggesting that pro-inflammatory molecules’ levels increase with DR progression: in particular, serum concentration of circulating cytokines such as TNF-alpha, VEGF, IL-1beta and IL-6 seem to be associated with DR severity and not only with the presence of DR (68-73). Also elevated systemic neutrophil count was found to be associated with the presence and severity of DR, indicating that neutrophil-mediated inflammation may play a role in DR pathogenesis (74). In the eye, increased expression of vitreous IL-8 seems to correlate with worse visual acuity in diabetic patients in one study (75), while another study found an increased aqueous concentration of IL-8 in severe NPDR, suggesting that inflammation may contribute to the development of neovascularization (76). IL-6 aqueous concentration has a positive correlation with macular thickness, indicating that IL-6 may play a role in the development of DME (77). On the contrary, both circulating and aqueous levels of IL-10, a cytokine able to down-regulate T lymphocytes helper 1 response and VEGF, are decreased in DR and DME (78,79) and one recent study demonstrated a correlation between low circulating IL-10 concentration and a cystoid pattern of DME (80) (Table 1).
table1
Table 1
| Biochemical biomarkers | Associated features | References |
|---|---|---|
| Cellular | ||
| VCAM-1, ICAM-1, selectins | Leukocyte adhesion | ( |
| TNF-alpha, IL-1, 6 and 10, MCP-1 and 2, VEGF, IFN-gamma, IP-10 | Microglia activation | ( |
| GFAP, AQP4 | MCs activation | ( |
| Clinical | ||
| TNF-alpha, VEGF, IL-1beta, IL-6 | DR progression | ( |
| IL-8 | Worse functional outcome* | ( |
| IL-6, IL-10 | DME and SND onset | ( |
*, worse visual acuity after vitrectomy performed for PDR. MCs, Müller cells; DME, diabetic macular edema; SND, serous neuroretinal detachment, PDR, proliferative diabetic retinopathy.
Therefore, there are many systemic and local (vitreous/aqueous) inflammatory molecules upregulated at any stage of DR. All of them interact to create a pro-inflammatory environment that contributes to occurrence, maintenance and progression of retinopathy.
Clinical biomarkers of inflammation in DR
A circulating molecule has to be highly specific to the retina to be a reliable biomarker of retinal diabetic disease rather than a marker of systemic disease (64). Aqueous or vitreous biomarkers are more specific but also more difficult to obtain needing an invasive procedure (in particular to collect a sample of vitreous fluid). Therefore, thanks to the great advances in retinal imaging technologies of the last years, a new concept of non-invasive “imaging biomarker” of retinal inflammation has emerged and has made its way to the study of patients with diabetes (64). Clinical research has consequently developed great interest in finding specific retinal parameters of the retinal inflammatory condition in DR and DME and there is a growing body of scientific evidence on the importance of this topic. Nowadays, proposed imaging biomarkers of inflammation in DR include subfoveal neuroretinal detachment (SND) and hyperreflective retinal spots/foci (HRS) visible on SD-OCT and increased foveal autofluorescence (iFAF) visible on fundus autofluorescence imaging (56,58,82-86) (Figure 1). In addition, an increase in thickness of the inner nuclear layer was described on SD-OCT in patients with NPDR and this may represent a clinical sign of MCs activation due to hypertrophy of these cells (11). Hyperreflective foci were described on SD-OCT also in the vitreous of patients with diabetes, with the number increasing as the stage of DR progresses (87,88). Previous studies had demonstrated in patients undergoing vitrectomy for complicated PDR that there was an increased number of lymphocytes that was correlated to disease severity (89,90). Thus, hyperreflective foci detected with SD-OCT in the vitreous of these patients were interpreted as inflammatory cells infiltrating the vitreoretinal interface and vitreous gel (87) (Table 2).
figure1
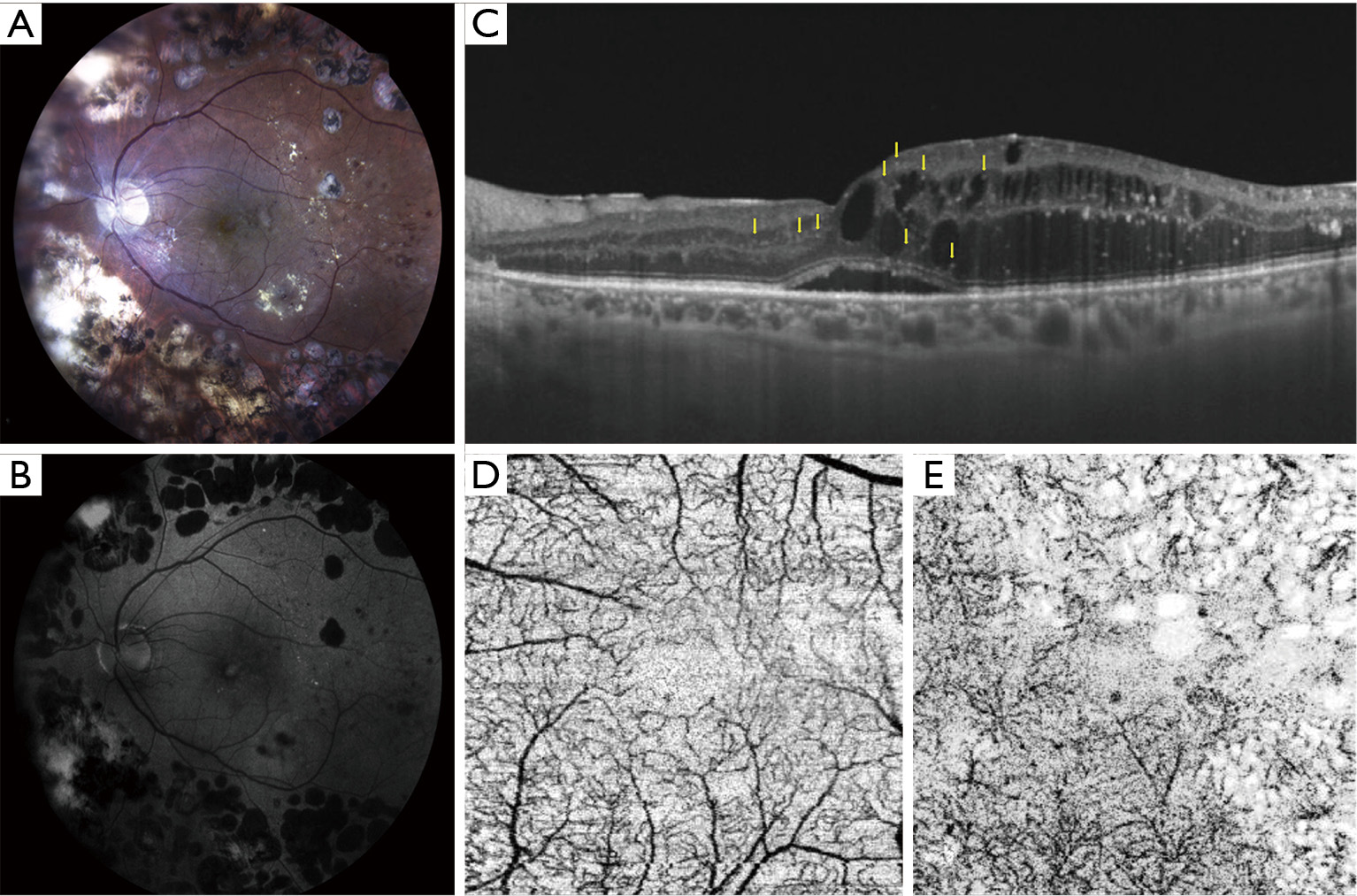
table2
Table 2
| Clinical biomarkers | Imaging technique | References |
|---|---|---|
| Subfoveal neuroretinal detachment | OCT | ( |
| Hyper-reflective retinal spots/foci | OCT | ( |
| Increased fundus autofluorescence | FAF | ( |
| Vitreous hyper-reflective foci | OCT | ( |
| Increased INL thickness | OCT | ( |
OCT, optical coherence tomography; FAF, fundus autoflurescence in the fovea; INL, inner nuclear layer.
SND
SND is detected on OCT as a hyporeflective area beneath the neuroretina (extracellular fluid accumulation between the outer segments of photoreceptors and the retinal pigment epithelium) in approximately 15-30% of eyes with DME (85,101-104). The presence of SND has been associated with higher levels of local inflammatory molecules, in particular IL-6 (85) and in the past it was considered to correlate with a poorer visual outcome (91,92). SND is more likely to develop in the presence of increased choroidal thickness (increased choriocapillaris permeability and outer BRB impairment) (93,94), disrupted external limiting membrane (ELM) (94,101) and in association with a significantly increased number of HRS, another clinical sign of local inflammation (83,94).
Hyper-reflective retinal spots
HRS are not a specific sign of DR and have been described in other chorioretinal diseases, such as age-related macular degeneration (AMD), retinal vein occlusion and recently uveitis (56,95,96,105-110). In all these conditions HRS are visible in both inner and outer retina and also near, in the walls or inside the lumen of retinal cysts (56,95,96,105-109). Histologic evidence from human donor eyes with AMD and acquired vitelliform lesions demonstrated that HRS visualized on SD-OCT may originate from both anteriorly migrated retinal pigment epithelium cells and lipid-filled cells (thought to correspond to activated microglia) (97,111,112). HRS are increased in number in patients with diabetes (in both preclinical and early clinical DR) versus normal subjects and they are thought to represent aggregates of activated microglial cells that progressively migrate from the inner to the outer retina, confirming their role as inflammatory biomarkers (56). Further evidence supporting this hypothesis came from a recent study demonstrating a positive correlation between aqueous concentration of soluble CD14 (a cytokine associated with immune response, expressed in microglia, monocytes and macrophages) and the number of HRS in DME (98). In particular, HRS related to microglia activation seem to have specific characteristics, such as small dimension (<30 μm), reflectivity similar to nerve fiber layer, absence of back-shadowing, and location in both inner and outer retina; additionally, they do not correspond to any specific lesion on fundus examination (56,58).
Increased fundus autofluorescence in the fovea
iFAF was described in a large proportion of eyes with DME (82,84,99) and is correlated to reduced retinal sensitivity determined with microperimetry, indicating an impaired neurosensory retina function in that area (82). In DME, it is thought that areas of iFAF are caused by accumulation of oxidative products induced by activated microglial cells (100,113), thus suggesting that also iFAF may be considered as an imaging biomarker of microglial activation in DME (82).
Conclusions
Even if not all mechanisms are still fully elucidated, actual evidence highlights the important role of inflammation in DR pathogenesis and progression, and DR is considered a chronic, low-grade inflammatory disease. In particular leukocyte recruitment and adhesion to retinal vessels and glial cell activation are recognized as early events occurring in diabetic retina dysfunction, even before the onset of clinically evident signs of retinopathy.
Current available treatment options for DR (intravitreal injections of anti-VEGF or corticosteroids, laser photocoagulation, vitreoretinal surgery) are applicable only at advanced stages of disease (DME, severe NPDR, PDR) (64,114-117). In early stages the only therapeutic strategy that physicians can offer is the control of modifiable risk factors for DR such as glycaemia and systemic blood pressure (64,117). Therefore, there is an urgent need for non-invasive early-detection molecular and clinical biomarkers of subclinical and early DR that can help in DR management before irreversible damage occurs.
Measuring local and systemic biomarkers of inflammation may become a useful tool to differentiate patients with diabetes on the basis of their risk of disease progression, however, further validation is needed. In addition, the increasing understanding on inflammation involvement in DR is stimulating the interest in targeting specific inflammatory pathways to improve DR prevention and care, even if specific interventions are still not part of a routine clinical practice and further work is needed on this front.