A review of corneal nerve and limbal stem cell
关键词
摘要
全文
HIGHLIGHTS
The cornea is a transparent tissue that serves as the main refractive element of the eye ball, and consists of five layers: the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium[1,2]. Limbal epithelial stem cells (LESCs), residing in the basal epithelial layer of the Palisades of Vogt found in the corneal limbus located between cornea and scleral, are believed to be crucial for the continuously turnover of corneal epithelium[3-5]. The proliferation, migration, and differentiation of the LESCs are modulated by unique physical and chemical futures contained within the microenvironment known as limbal niche, which composed of nerve terminals, cells, extracellular matrix, vasculature and signaling molecules[5,6]. The maintenance of LESCs and corneal epithelial homeostasis has been found to be orchestrated by core transcription regulatory circuitries (CRCs), with RUNX1 and SMAD3 being required for the maintenance of corneal epithelial identity and homeostasis[7]. Current data suggest that LESCs may comprise two coexisting populations, termed the “outer” and “inner” limbus, localized in separate and well-defined sub-compartments[8]. The primitive population of quiescent outer LESCs participates in wound healing and boundary formation and is regulated by T cells, which serve as a niche. In contrast, the inner peri-corneal limbus hosts active LESCs that maintain corneal epithelial homeostasis[9].Another study reported that corneal stem cells are not only located in the limbus but also distributed throughout the entire ocular surface in other mammals[10]. However, this does not explain the phenomena that damage to limbal area, such as chemical burn, surgeries, and contact lens use, leading to the limbal stem cell deficiency (LSCD), usually results in the invasion of conjunctival epithelium onto the corneal surface and corneal neovascularization in human[11]. Therefore, most researchers still focus on the view that corneal epithelial stem cells reside in the limbal area.
LESCs are considered to be a group of cells exhibiting the special characteristics of mitotic inactivity and high proliferative potential, which is activated during the transient amplifying stage. They divide asymmetrically to produce one stem cells and one transient amplifying cell ( TAC, a cell capable of multiple but limited cellular division) to maintain the stem cell population. TACs migrate to the central cornea along the basement membrane and responsible for the turnover of the epithelium[12]. The balance of this process is modulated within the limbal niche. Most research groups have focused on the studying cells (e.g. mesenchymal stem cells), extracellular matrix, and biological factors (e.g. hemoderived factors, soluble factors/cocktails) that function to maintain the LESCs population and reconstruct the limbal niche based on these theories[13-19]. However, increasing evidences has demonstrated that nerve endings play an important role in the regulation of the epithelial stem cell niche[20-22].
Corneal nerves plays a critical role in the regulation of corneal epithelial homeostasis by stimulating proliferation, regeneration, differentiation, and possibly migration[23]. Malfunction of nerves, such as in diabetes mellitus, ocular herpes simplex, neoplasia, and ophthalmic surgery, typically leads to the neurotropic keratopathy, which exhibits symptoms including persistent corneal epithelial defects, stromal opacification, stromal melting, and neovascularization. This phenomenon has been demonstrated through a series of experimental models involving cornea denervation[24-26]. Furthermore, growing evidence has shown a close correlation between corneal basal cell density (BCD) and nerve parameters in models of LSCD. Increasing severity of LSCD is accompanied by a decline in the density of the central corneal sub-basal nerve plexus density (SND)[27-29]. Moreover, in an animal model of ocular denervation, the number and function of corneal stem/progenitor cells were significantly reduced[30,31]. Biological molecules, such as neuropeptides, neurotransmitters and neurotrophic factors, play pivotal role in modulating the phenotype of LESCs, which are responsible for corneal epithelium homeostasis. This paper will review recent studies on how does these nerve-derived molecules function in this process and provide clear directions for future research.
ANATOMY OF LESCs NICHE AND CORNEA NERVE
LESCs niche and their biological functions
LESCs are considered to reside in the special area of the limbus, defined as the limbal palisades of Vogt (POV) . Within this area, numerous limbal epithelial crypts (LECs) are found, which extend radially from the limbal palisades into the conjunctival stroma[32]. As reported, LECs were uniformly distributed around the corneal circumference and are considered to be the potential stem cell repositories[33]. The anatomy of POV and LECs, together with cellular elements (e.g. immune cells, mesenchymal stem cells, and melanocytes), vascular and nerve terminals, extracellular matrix (ECM), and signaling molecules, comprises a niche that is related to the maintenance of the stem cell functions for corneal epithelial homeostasis[34,35]. The process of corneal epithelium turnover can be summarized as follows: LESC located in the basal epithelial layer of LEC divide symmetrically into two identical cells or asymmetrically into one LESC and a TAC; then, the TACs are divided into post-mitotic cells (PMCs) as they migrate centripetally and differentiate into terminally differentiated cells (TDCs)[36](Fig 1).Figure 1 Hypothetical scheme of limbal stem cell niche[36]
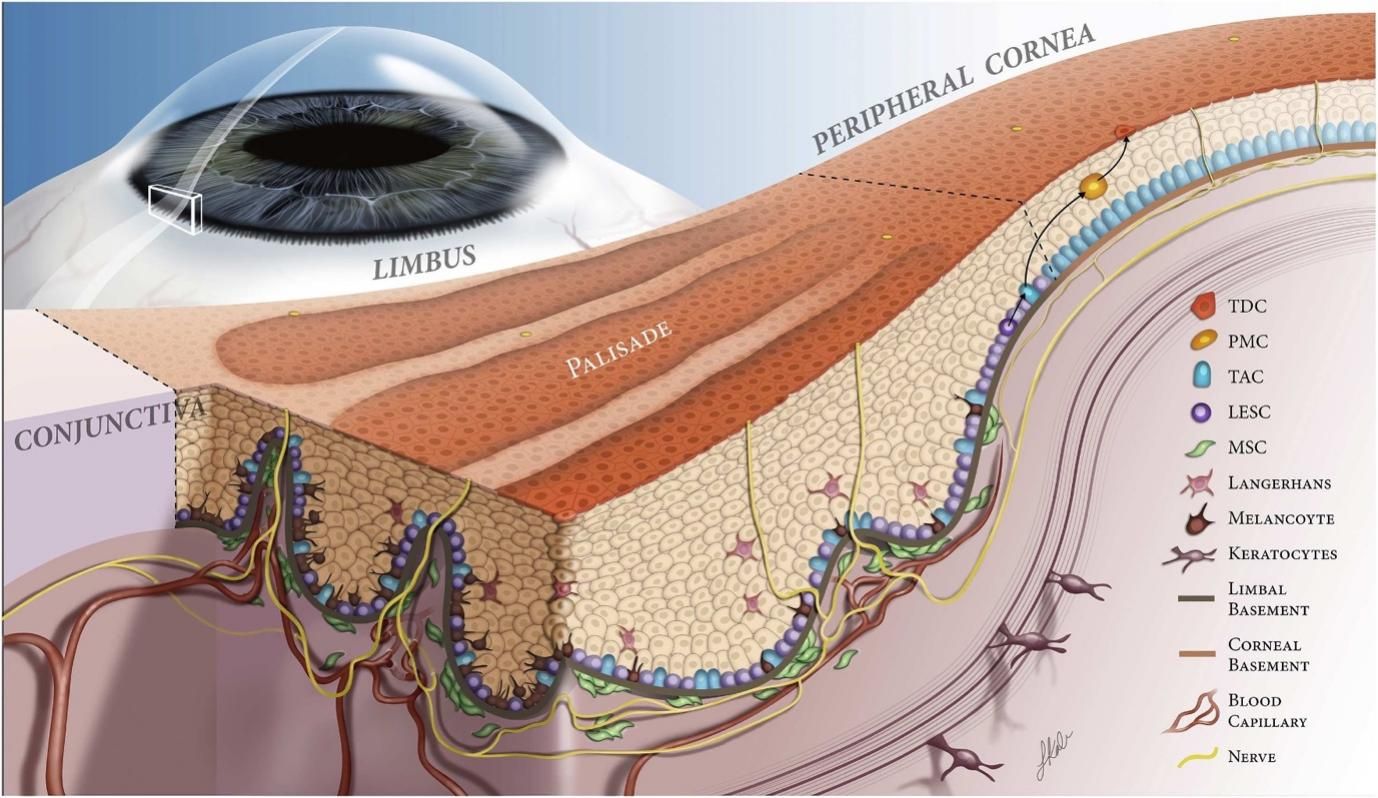
TDC, terminally differentiated cell; PMC, post-mitotic cells; TAC, transient amplifying cell; LESC, limbal epithelial stem cell; MSC, mesenchymal stem cell.
Nerves in the cornea
The cornea is innervated by both the sensory and autonomic nervous systems. The human cornea receives most of its sensory innervation from two or three long ciliary nerves, which originate from the nasociliary nerve, a branch of the first (ophthalmic) division of the trigeminal nerve. Before reaching the corneoscleral limbus, nerves fibers repeatedly bundle together and anastomose extensively with branches of the short ciliary nerves, distributing uniformly around the corneal circumference and entering the cornea radially from all directions[37,38].Upon entering the corneoscleral limbus, the parent nerve gives rise to highly tortuous nerves that ultimately terminate in CNE, which are encapsulated compact structure often referred to as “receptors” or “ corpuscles” (Fig 2).The CNEs are predominantly arranged in clusters, located in a close proximity to the limbal POV, and completely surrounded by limbal epithelial crypts (LECs), which are considered as stem cell niches (Fig 3)[21]. This interesting and unique structure suggests that CNE may play a critical role in the maintenance of limbal stem cell niche.Figure 2 The whole-mount acetylcholinesterase-stained cornea
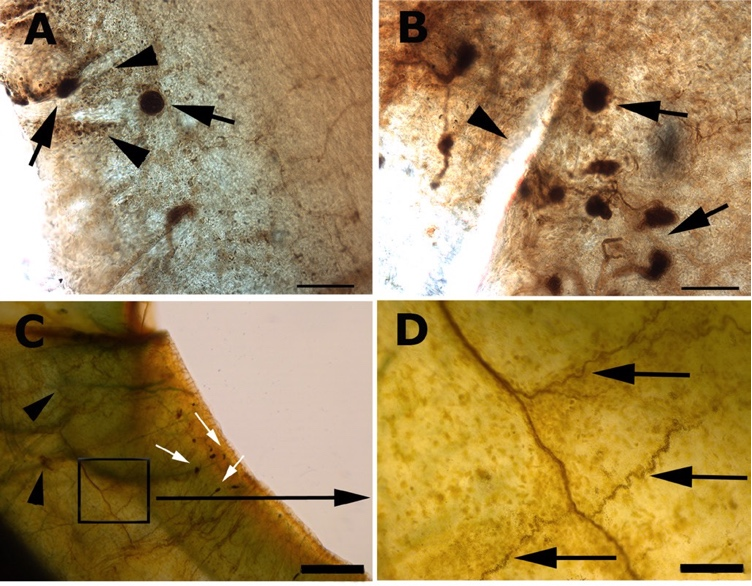
(A,B) CNE (black arrows) distributed around limbal POV (arrowheads). (C) Nerve fibres in the stroma (arrowheads). White arrows point to CNE in the peripheral cornea. (D) The deep stroma nerve gives rise to tortuous nerve branches (arrows). The tortuous nerves terminate in CNE as seen in C. Bar=100 μm (A,B), 500 μm (C), 100 μm (D), 10 μm (E), 25 μm (F), 120 μm (G) and 100 μm (H) [21]
Figure 3 Acetylcholinesterase prestained sections of limbus demonstrating CNE (arrows and arrowheads) completely wrapped by LECs. Bar=50 μm (A–D) [21]
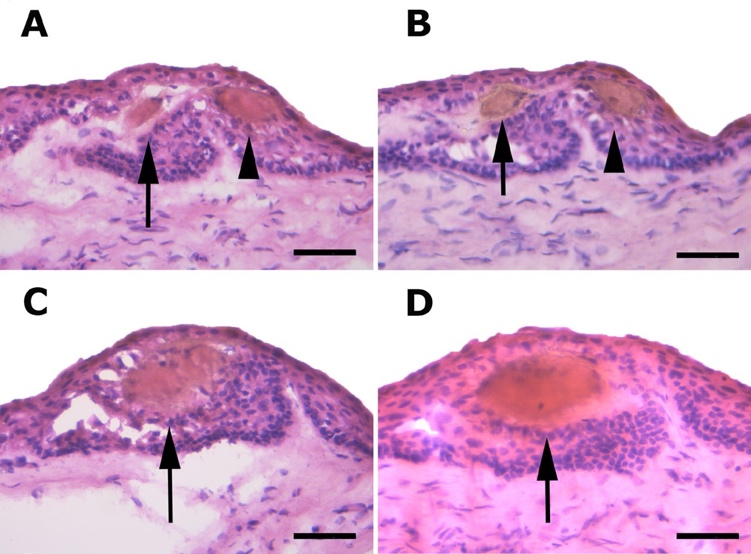
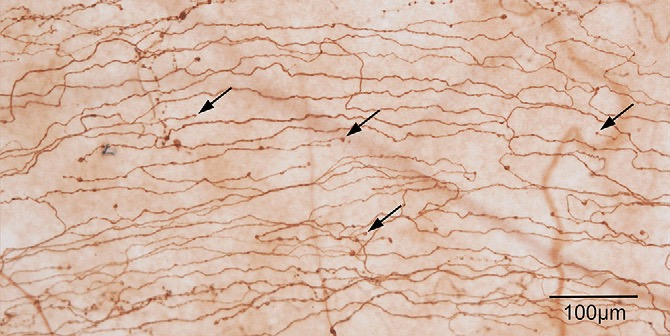
Figure 4 Subbasal nerves in the peripheral cornea. Arrows, bulbous nerve endings in the basal epithelial cell layer[48]
NERVE DERIVED BIOLOGICAL MOLECULES
Substance P
Substance P (SP), an 11-amino acid peptide encoded by the Tachykinin Precursor 1 (TAC1) gene, belongs to the tachykinin neuropeptide family and is highly expressed in both the peripheral and central nervous systems. It is secreted by specific neuronal cells as well as non-neuronal cell type, such as epithelial cells and immune cells, and exerts its functions by binding to the G protein-coupled neurokinin receptors (NKRs), which are expressed by many cell types in the body. There are three types of NKRs: NK1R, NK2R, and NK3R. Among members of the NKR family, neurokinin receptor 1(NK1R) has the highest affinity for SP[50], and is expressed in corneal epithelial cells and keratocytes[51]. The SP-NK1R signaling pathway is believed to mediate a diverse set of pathways, including those involved in corneal neovascularization, inflammation,cell proliferation, migration, apoptosis, stem cell mobilization, and has profound implications for wound healing and inflammatory modulation[50]. NK1R is proven to be expressed on the cell membrane of corneal epithelial stem cells, supporting the key role of SP and nerves in stem cell pathophysiology. It is reported that SP ablation or NK1R blockade significantly enhances epithelial wound healing and corneal transparency[52]. Additionally, excessive expression of SP can induce senescence and exhaustion of residual stem cells through activation of NK1R, which is associated with LSCD. SP aggravates corneal LSCD through stimulation of the mammalian target of rapamycin(mTOR) pathway, eventually leading to accelerated corneal epithelial cell senescence. It is identified that the mTOR signaling, as a key driver of stem cell activity, participates in corneal epithelial cell proliferation and differentiation[53].It is widely reported that SP is a key mediator of neurogenic inflammation through the enhancement of the microvascular permeability, vasodilatation, and plasma extravasation[54]. However, SP plays an indispensable role during wound healing process by functioning as an injury-inducible messenger[55] and exerts an activity in maintaining corneal epithelium homeostasis[56]. It has been revealed that the effect of SP on epithelial wound closure is time and concentration dependent[57]. In addition, Romina hypothesized that an early, controlled release of SP is beneficial, while excessive amounts may impair healing[58]. The motility of many cells types is enhanced by SP through different signaling pathways, such as mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinase-Akt, protein kinase C and epidermal growth factor receptor (EGFR) pathways[56,59]. In a diabetic model, SP was found to promote corneal epithelial wound healing by stimulating the reactivation of Akt, EGFR and Sirt1,thereby alleviating the epithelial lesions[56].The corneal sensitivity of diabetic mice was also restored by the activity of SP-NK1R signaling pathway, which was compatible with the decrease in the corneal nerve terminal density observed in NK1R knockout mouse[60]. Unlike the diabetic model, SP needed to act synergistically with insulin-like growth factor-1(IGF-1) to promote corneal epithelial defect closure. This combined effect of SP/IGF-1 was mediated by NK-1R, but not by NK2R and NK3R[61]. Moreover, the SP-NK1R interaction has been shown to inhibit the apoptosis of the corneal epithelial cells in vitro[62]as well as regulate the expression of E-cadherin and ZO-1 tight junction proteins in the corneal epithelium[63,64], and exhibited an important role in the maintenance of the corneal epithelium homeostasis.Several reports have demonstrated that SP can enhance corneal wound healing both by regulating the proliferation, migration and differentiation of LESCs as well as the mobilization of the MSCs[64-68]. Furthermore, additional studies should be conducted to evaluate this hypothesis.
Calcitonin gene-related peptide(CGRP)
CGRP, a 37-amino-acid neuropeptide expressed by trigeminal ganglion neurons and corneal nerves, is widely distributed in the nervous system alongside SP. Previous studies have investigated the expression of CGRP in the cornea sensory nerve across in a wide range of animal species. CGRP plays a crucial role in immune reactions by regulating immune cells such as macrophages, neutrophils, dendritic cells[69]. In a study of corneal inflammation induced by Pseudomonas aeruginosa (P. aeruginosa), corneal sensory neuron activation, macrophage recruitment, and up-expression of CGRP were observed. The synergistic effect between sensory neurons and macrophages promotes the release of CGRP. CGRP inhibits corneal inflammation and promotes the transformation of macrophages to the M2 phenotype through the PI3K/AKT signaling pathway[70].After being applied topically to the ocular surface in a corneal epithelial wound model, CGRP was found to enhance the healing rate of corneal epithelial cells through a cAMP-dependent effect on actin filament polymerization, which is involved in the cell migration[71]. Recent research has demonstrated the CGRP derived from trigeminal ganglion sensory neurons and corneal nerves promotes corneal angiogenesis. Moreover, blocking the signaling of nerve-derived CGRP inhibits corneal angiogenesis[72]. Paradoxically, in a model of corneal injury induced by SM, the expression of CGRP increased while the number of limbal stem cell declined and nerve damage occurred. The report speculated that CGRP might be responsible for changes in the LESC niche , as it has been shown to interact with corneal epithelial cells in vitro to release interleukin-8, which acts as a chemoattractant for neutrophils into the inflammation area, a process related to the “neurogenic inflammation” [73]. In a study of corneal injury induced by lacrimal gland excision, the expression of CGRP was observed in isolectin B4 (IB4) -positive corneal neurons. This response may initially facilitate axonal regeneration;however, the increased expression of CGRP is also likely to contribute to persistent corneal pain[74]. Furthermore, CGRP’s role in neuroplasticity and hypersensitivity explains its close association with photophobia, a common symptom in dry eye disease , migraine and traumatic brain injury[75]. Further data should be collected to evaluate the precise role that CGRP plays in maintenance corneal epithelial homeostasis.
Melanocyte-stimulating hormones (MSH)
MSH,a family of peptide hormones and neuropeptides consisting of α-MSH, β-MSH, and γ-MSH, is generated from different cleavages of the proopiomelanocortin(POMC) protein. It is mainly secreted by melanocytes, neurons or anterior lobe of the pituitary gland, and functions to stimulate the production and release of melanin in skin and hair, suppress appetite, and contribute to sexual arousal in the hypothalamus[76]. α-MSH, a 13-amino-acid peptide, is widely distributed in the skin and cornea[77]. It is also well-known for its roles in metabolic regulation, neuroprotection, inflammation suppression, and anti-angiogenic effects[78-81], mediated through binding to the melanocortin receptors, which consist of 5 subtypes (MC1R-MC5R) belonging to the G protein-coupled receptor(GPCR) family[76].In a study of scopolamine-induced dry eye syndrome, α-MSH was found to restore ocular surface functions by upregulating EGFR expression in the JAK-STAT signaling pathway[77].Melanocytes, one of the most important cells in the LESC niche, are responsible for pigmentation in the perilimbal area, constructing an anti-oxidant system for LESC protection[82,83]. The α-MSH/MC1R signaling pathway plays vital roles in bulge melanocyte stem cells and melanin synthesis[84]. Additionally, α-MSH/MC1R signaling is critical for corneal endothelial cell function and graft survival after corneal transplantation[85]. Therefore, corneal nerve might play a role in the LESC niche environment through the regulation of α-MSH/MC1R signaling pathway.
Acetylcholine (ACh)
Acetylcholine, a choline molecule acetylated at the oxygen atom, is synthesized mainly in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA. It is regarded as a classical neurotransmitter that is widely distributed in central nervous system, neuromuscular junctions, and autonomic nervous system[86]. However, increasing evidence has demonstrated that ACh is also synthesized by a majority of human cells, such as cornea epithelium[87], and exerts its functions in cellular process (e.g. proliferation, differentiation, adhesion, migration, secretion, etc) , anti-inflammation, and stimulating wound healing (e.g. skin, cornea) by binding to nicotinic and muscarinic receptors(nAChRs and mAChRs, respectively)[88-92]. nAChRs on nerve endings in the nose initiate a reflex arc resulting in instantaneous tear secretion, which represents a novel approach to treat dry eye disease by increasing endogenous tear production[93,94]. Furthermore, the M3 receptor plays a pivotal role in tear production, and its absence leads to ocular surface changes typical of dry eye disease in advanced age[95].nAChRs (α1-10, β1-4, γ, δ and ε subunits) and mAChRs (M1-M5) have been found to be expressed in many types of cells, including corneal epithelial cells. The nAChRs are directly linked to icon channels that mediate the influx of Na+ and Ca2+ and efflux of K+, and they can also activate the downstream signaling pathways by modulating the activities of protein kinases and phosphatases[96]. Activation of the nAChR and mAChRs pathways promotes epithelial cell division and re-epithelialization after corneal abrasion[97,98]. This biological function, mediated by the cytotransmitter ACh-based signal transduction, is not only regulated by the cholinergic nervous system, but also by the autocrine/paracrine in the corneal epithelium[99]. Nevertheless, the role of ACh and its downstream signaling pathways in the process of corneal epithelium re-epithelialization remains unknown. The processes and mechanisms of cholinergic modulations in stem cells are complex, and further studies are needed to elucidate the mechanisms underlying this biological function and to determine whether it plays a role in regulating corneal epithelial stem cell functions and corneal epithelium homeostasis.
Catecholamines
Catecholamines, including epinephrine (E), norepinephrine (NE)and dopamine(DA), are primarily synthesized by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system. These compounds originate from the amino acid tyrosine. The catecholamine-secreting cells initially convert tyrosine to L-Dihydroxyphenylalanine(L-DOPA), which is subsequently transformed into DA. DA is then further metabolized into NE by dopamine β-hydroxylase or into E by phenylethanolamine N-methyltransferase[100]. NE and E bind to the 7-transmembrane, G protein-coupled receptors known as adrenoceptors, which comprise three major types: α1, α2 and β1-3. Adrenoceptors are widely expressed in both the central nervous system and peripheral tissues, and they exert their functions on cellar processes primarily through cAMP-PKA downstream pathways.In the cornea, catecholamines and adrenoceptors are believed to play a role in the proliferation of corneal epithelial cells. NE, the principal neurotransmitter produced and released by sympathetic nerves, is significantly more abundant than E in rabbit and human corneas, while DA is found to be the most prevalent catecholamine in the central and intermediate segments of the cornea[101]. It has been reported that NE is directly or indirectly associated with the onset and exacerbation of corneal inflammation[58,102,103]. Human corneal limbal epithelial cell sheets cultured on carboxymethyl cellulose (CMC)-DA-coated substrates have been safely transplanted in a rabbit animal model of LSCD[104]. Notably, there is no evidence to suggest that the E is present in the human cornea. The α2 adrenoceptors are primarily located in the intercellular spaces of the epithelial basal layer, particularly in the peripheral anterior cornea[105]. The concentration gradient of NE decreases from the periphery to the center of the cornea[101]. Both E and α2 adrenoceptor exert their effects on corneal epithelial cells by regulating the cAMP-PKA pathway, which plays a crucial role in corneal wound healing and overall homeostasis[106,107]. Furthermore, activating the DA receptors (D1/D2) expressed in corneal epithelial cells and endothelial cells in the wounded cornea increases the nerve growth factor (NGF) and corneal nerve density, thereby enhancing corneal epithelial healing[108,109]. This suggests that DA may have indirect function in maintaining corneal epithelium homeostasis through the NGF signaling pathways.
Ciliary neurotrophic factor (CNTF)
CNTF is a protein encoded by the CNTF gene in humans. It belongs to the interleukin 6(IL-6) family cytokines, which includes IL-6, IL-11, CNTF, leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotrophin 1 (CT-1), cardiotrophin-like cytokine (CLC), and IL-27[110]. When induced pluripotent stem cells(iPSCs) respond to the electrical activity of their microenvironment, they increase endogenous CNTF release to enhance neuronal differentiation and facilitate the rapid conversion of iPSCs. CNTF can induce adipose-derived mesenchymal stem cells (AMSCs) to exit the cell cycle, undergo mitosis, and initiate the expression of neuron markers[111]. CNTF has been shown to improve the generation of cornea nerve fibers[112], but both CNTF and its receptor, CNTFRα, are hardly expressed in normal human cornea[113,114]. Interestingly, in mouse models, CNTF has been found to improve corneal epithelial wound healing and promote the proliferation of corneal epithelial stem/progenitor cells by the activation of STAT3 signaling pathway, a transcription factor that regulates cellular processes including apoptosis, proliferation, migration, and survival[115]. However, the mechanism underlying this biological function, how CNTF regulates the STAT3 signaling pathway and whether there are any differences in CNTF/ CNTFR expression in cornea epithelium among different species remains unknown.Nerve growth factor (NGF)
NGF is a well-known neurotrophic factor and neuropeptide taht is widely distributed in the nervous system and major cell types throughout the body. It plays an important role in development and survival of sensory neurons[116], as well as in the cellular process such as proliferation, differentiation, survival, and apoptosis. It also regulates the immune system[117], accelerates wound healing in the skin and cornea[118,119], and exhibits insulinotropic, angiogenic[120], and antioxidant properties[121].NGF has been found to orgiginate from both corneal nerves and limbal basal epithelium, where it promotes sustained corneal epithelium wound healing, the recovery of corneal sensitivity and cornea nerve regeneration[122,123]. As a rhNGF, Cenegermin has shown beneficial effects on neurotrophic keratopathy, with effects persisting several months after an 8-week course of topical rhNGF treatment[124]. NGF has been found to extend the lifespan of LESCs in vitro, promoting their proliferation, colony-forming efficiency, and maintenance of phenotype ( as evidenced by ΔNp63α and ABCG2 expression)[1]. The NGF/TrkA signaling pathway promotes the migration and proliferation of limbal epithelial cells by activating various signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk), and phospholiase C-γ (PLC-γ)[125].Therefore, NGF is likely to play an important role in regulating the LESC niche. Recently, topical recombinant human nerve growth factor (rhNGF) has been demonstrated to be a safe and effective agent for promoting the healing of moderate-to-severe neurotrophic keratitis[126,127]. The expression of microRNAs in human epithelial corneal cells after time-dependent rhNGF treatment has also been analyzed, revealing a potential strong impact of miRNAs on the modulation of specific NGF-induced cellular responses[128]. Hsa-miR-143-3p has been shown to play a role in the maintenance of CESCs through downregulating of key proteins involved in Wnt and MAPK signaling[129]. However, the mechanism by which NGF regulates the LESC phenotype requires further investigation.
Melanocyte-stimulating hormones (MSH)
MSH,a family of peptide hormones and neuropeptides comprising α-MSH, β-MSH, and γ-MSH, originates from different cleavages of the proopiomelanocortin(POMC) protein. It is mainly secreted by melanocytes, neurons or the anterior lobe of the pituitary gland, and it functions to stimulate the production and release of melanin in skin and hair, suppress appetite, and contribute to sexual arousal while in the hypothalamus[76]. α-MSH, a 13-amino-acid peptide, is found widely distributed in skin and cornea[77]. It is also well-recognized for its roles in metabolic regulation, neuroprotection, inflammation suppression, and anti-angiogenic activities[78-81], mediated through binding to the melanocortin receptors, which consist of 5 subtypes (MC1R-MC5R) belonging to the G protein-coupled receptor(GPCR) family[76].In a study of scopolamine-induced dry eye syndrome, α-MSH restored ocular surface functions by upregulating EGFR expression via the JAK-STAT signaling pathway[77].Melanocytes, one of the most important LESC niche cells, are responsible for the pigmentation in POV, thereby establishing an antioxidant system for LESC protection[82,83]. The α-MSH/MC1R signaling pathway plays vital roles in bulge melanocyte stem cells and melanin synthesis[84]. Furthermore, α-MSH/MC1R signaling is critical for corneal endothelial cells function and graft survival after corneal transplantation[85]. Thus, corneal nerve might play a role in LESC niche environment through the regulation of α-MSH/MC1R signaling pathway.
A brief introduction, expression site, and possible regulatory role of the seven nerve-derived biological molecules mentioned above have been summarized in Table 1 for reference.
Table 1 A brief introduction, expression site, and possible regulatory role of the seven nerve derived biological molecules
|
biological molecules |
Brief introduction |
Expression site |
Possible regulatory role |
|
11-aminoacid peptid,belongs to the tachykinin neuropeptide family |
highly expressed in the peripheral and central nervous systems |
SP-NK1R signal pathway:involved in corneal neovascularization, inflammation,cell proliferation, migration, apoptosis, stem cells mobilization and wound healing and inflammatory modulation. mTOR pathway:accelerated corneal epithelial cell senescence. Akt, EGFR and Sirt1 pathway:promoted the corneal epithelial wound healing,alleviated the epithelial lesions. SP/IGF-1:promoted corneal epithelial defect closure. Nanog and Wnt/ β-catenin signaling pathway:improve the corneal wound healing both by regulating the proliferation,migration and differentiation of LESCs and the mobilization of the MSCs.
|
|
|
Calcitoningene-related peptide(CGRP) |
37-amino-acid neuropeptide |
widely distributed in the nervous system in accordance with SP |
PI3K/Akt and p38MAPK signaling pathway:homed of human umbilical cord mesenchymal stem cells (HUMSCs) ,inhibited corneal inflammation and promoted the transformation of macrophages to the M2 phenotype. nerve-derived CGRP signaling pathway:corneal angiogenesis. |
|
Melanocyte-stimulating hormones (MSH)
|
|
widely distributed in skin and cornea |
JAK-STAT signaling pathway:restored ocular surface functions by upregulating EGFR expression. α-MSH/MC1R signaling pathway:critical for corneal endothelial cells function and graft survival after corneal transplantation. |
|
Acetylcholine (ACh)
|
choline molecule that has been acetylated at the oxygen atom,synthesized mainly in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA |
widely distributed in central nervous system, neuromuscular and autonomic nervous system |
nAChRs directly linked to icon channels:mediate the influx of Na+ and Ca2+ and efflux of K+. downstream signaling pathway:activated by modulating the activities of protein kinases and phosphatases. M1,M3 and M5 receptors acoupled with Gq proteins:activated protein kinase C (PKC) by elevating intracellular Ca2+ and diacylglycerol. M2,M4 receptors coupled with Gi/o proteins: inhibited protein kinase A by diminishing adenylyl cyclase activity. nAChR and mAChRs pathway:promoted the epithelial cells division and re-epithelialization after corneal abrasion,modulating the intestinal crypt stem cells proliferation and differentiation by acting on intracellular pathways such as Wnt or trans-activate EGER. |
|
Catecholamines |
including epinephrine (E), norepinephrine (NE)and dopamine(DA), are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system
|
largely expressed both in the central nervous system and in peripheral tissues |
cAMP-PKA downstream pathway:exerted their functions on cell process,corneal wound healing and overall homeostasis. NDF signaling pathway:ctivated the DA receptor (D1/D2) that expressed in corneal epithelial cells and endothelial cells in the wounded cornea increased the NDF impression and corneal nerve density. |
|
Ciliary neurotrophic factor(CNTF) |
protein that in humans encoded by the CNTF gene,belongs to the interleukin 6(IL-6) family cytokines consisting of IL-6, IL-11, CNTF, leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotrophin 1 (CT-1), cardiotrophin-like cytokine (CLC), and IL-27 |
widely distributed in neurons, glial and Schwann cells throughout the central and peripheral nervous system |
induced pluripotent stem cells(iPSCs)’s response:increase endogenous CNTF release to enhance neuronal differentiation and the iPSCs’ rapid conversion CNTFRα:permitting the recruitment of glycoprotein 130 kDa(gp130) and LIF receptor membrane spanning signal transducing units CNTF:improve the corneal epithelial wound healing, and promote the proliferation of corneal epithelial stem/progenitor cells |
|
Nerve growth factor (NGF) |
neurotrophic factor and neuropeptide |
widely distributed in nervous system and major groups of cells in the body |
NGF/TrkA signal pathway:promote the migration and proliferation of limbal epithelial cells by inducing the activation of various signaling pathways, including phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt) and mitogen activated protein kinase (MAPK)/extracellular signal-regulated kinase (Erk) and phospholiase C-γ (PLC-γ) p75NTR:bind to and increase the affinity of TrkA for NGF as well as modulating its downstream signaling pathways,and downregulated upon the LESCs differentiation Wnt and MAPK signaling pathway:Hsa-miR-143-3p in the maintenance of CESCs through downregulation of key proteins involved in Wnt and MAPK signaling |
SUMMARY AND CONCLUSION
Corneal epithelium homeostasis is regulated by a complex biological process. Although some researchers suggest that corneal epithelial stem cells reside the entire ocular surface, most studies indicate that LESCs are responsible for the turnover of corneal epithelium. The niche in the limbal POV is the site where LESCs undergo processes such as proliferation, migration and differentiation. LESCs may comprise at least two types of cells: one that remains quiescent during corneal epithelial wounding, and another that becomes proliferative in response to such wounding. Corneal nerve terminals possess unique anatomy structures in the limbal POV and basal epithelial cells, and they exert crucial biological effects in the regulation of the LESC function and corneal epithelium homeostasis. A major group of neuro-derived biological molecules participate in this process through indirect modulation of the niche environment or direct regulation of LESCs functions.Substance P acts through the SP-NK1R signaling pathway, mTOR pathway, Akt, EGFR, and Sirt1 pathways, as well as the Nanog and Wnt/β-catenin signaling pathways. Calcitonin gene-related peptide (CGRP) is involved in the PI3K/Akt and p38 MAPK signaling pathways, as well as in nerve-derived CGRP signaling pathways. Melanocyte-stimulating hormones (MSH) function through the JAK-STAT signaling pathway and the α-MSH/MC1R signaling pathway. Acetylcholine (ACh) operates through nAChRs, which are directly linked to ion channels, and downstream signaling pathways, including M1, M3, and M5 receptors coupled with Gq proteins, and M2 and M4 receptors coupled with Gi/o proteins. Additionally, the nAChR and mAChRs pathways are involved. Catecholamines function through the cAMP-PKA downstream pathway and the NDF signaling pathway. Ciliary neurotrophic factor (CNTF) induces a response in induced pluripotent stem cells (iPSCs) through CNTFRα and CNTF. Finally, nerve growth factor (NGF) operates through the NGF/TrkA signaling pathway, p75NTR, and the Wnt and MAPK signaling pathways.Some of these neuro-derived molecules have been used clinically. For example, rhNGF has been successfully used for treatment of neurotrophic keratitis. Moreover, elucidating how corneal nerve exert their specific functions in regulating corneal epithelium homeostasis, especially in modulation of LESCs process, is important for the reconstruction of LESC niche and ocular surface. This review may provide some directions for future investigations on this topic and establish their clinical feasibility.Correction notice
NoneAcknowledgements
NoneAuthor Contributions
(I) Conception and design: Liqun Chen(II) Administrative support: Xianglong Yi
(III) Provision of study materials or patients: : Liqun Chen
(IV) Collection and assembly of data: : Liqun Chen
(V) Data analysis and interpretation: : Liqun Chen
(VI) Manuscript writing: All authors
(VII) Final approval of manuscript: All authors