Outcomes of Coaxial Micro-incision Phacoemulsification in Nanophthalmic Eyes: Report of Retrospective Case Series
阅读量:1210
DOI:10.3969 / j.issn.1000-4432.2015.03.002
发布日期:2025-01-07
作者:
Zi Ye ,Zhaohui Li
展开更多 '%20fill='white'%20fill-opacity='0.01'/%3e%3cmask%20id='mask0_3477_29692'%20style='mask-type:luminance'%20maskUnits='userSpaceOnUse'%20x='0'%20y='0'%20width='16'%20height='16'%3e%3crect%20id='&%23232;&%23146;&%23153;&%23231;&%23137;&%23136;_2'%20x='16'%20width='16'%20height='16'%20transform='rotate(90%2016%200)'%20fill='white'/%3e%3c/mask%3e%3cg%20mask='url(%23mask0_3477_29692)'%3e%3cpath%20id='&%23232;&%23183;&%23175;&%23229;&%23190;&%23132;'%20d='M14%205L8%2011L2%205'%20stroke='%23333333'%20stroke-width='1.5'%20stroke-linecap='round'%20stroke-linejoin='round'/%3e%3c/g%3e%3c/g%3e%3c/svg%3e)
关键词
coaxial micro-incision phacoemulsification
nanophthalmos
complication
摘要
Purpose: The surgical risk and complication rate after cataract
surgery are extremely high in patients with nanophthalmos. This study is designed to compare the visual and refractive
outcomes before and after coaxial micro-incision phacoemulsification
and evaluate postoperative complications.
Methods: Fifty nine patients (89 eyes) with axial length (AL)<21 mm diagnosed with nanophthalmos were enrolled in this
retrospective study. All patients underwent coaxial micro-incision
phacoemulsification and IOL implantation. The main
outcome measures included anterior chamber depth (ACD), anterior chamber volume (ACV), anterior chamber angle (ACA), intraocular pressure (IOP) and best corrected visual acuity (BCVA). Wilcoxon signed rank test or Mann-Whitney test, and Chi-square test and logistic regression analysis were performed
for statistical tests as appropriate.
Results: The median AL was 19.63 mm. Sixty-six eyes (74.16%) had a history of surgical intervention. Postoperative
ACD, ACV and ACA were increased significantly (all P<0.001), whereas postoperative IOP was decreased significantly. (P<
0.001) after surgery. Previous surgical intervention was related
to a reduction in the postoperative ACD and ACA (P<0.01), and both preoperative and postoperative IOP (P<0.001). Postoperative BCVA was improved in 94.38% of the cases. Intraoperative complications mainly included iridoschisis (6
eyes, 6.74%). Early postoperative complications included temporary
corneal edema (TCE) (23 eyes, 25.84%), anterior inflammatory
response (AIR) (19 eyes, 21.35%), cystoid macular
edema (CME) (14 eyes, 15.73%), and uveal effusion (4 eyes, 4.49%). Late postoperative complications included
CME (8 eyes, 8.99%), uveal effusion (8 eyes, 8.99%), malignant glaucoma (2 eyes, 2.25%) and posterior capsular
opacification (PCO)(10 eyes, 11.24%). The majority of
complications (80%) were successfully resolved by pharmacotherapy
or operation. The risk of surgical complications was
greater in patients with lower AL, ACD, ACV or ACA and
higher nuclear hardness or mean keratometry (Km).
Conclusion: With reasonable preoperative management, prudent
selection of the lens, rigorous surgical technique and
unerring cognition of potential complications, coaxial microincision
phacoemulsification lens surgery can be performed in
patients with nanophthalmos and yield favorable outcomes and
a low incidence of complications.
全文
Introduction
Nanophthalmos is a rare genetic disease characterized
by eyes with a short axial length (AL) less than
21 mm1. These small eyes have marked anatomic abnormalities, including a small cornea, shallow anterior
chamber, narrow angle, and high lens-to-eye
volume ratio. Patients with nanophthalmos are at
high risk for developing angle closure glaucoma2 which is hard to treat with traditional therapies. In
addition, pupillary block may be exacerbated by miotics,
thereby worsening the condition. Removal of
the lens was found to relieve anterior chamber
crowding, and thus is an optional treatment for
nanophthalmos-related glaucoma. Unfortunately, the
complication rate of the procedure is high3. Phacoemulsification
cataract surgery is increasingly being
performed for the management of cataract in
nanophthalmos4.
Currently, the outcome of phacoemulsification and intraocular lens (IOL) implantation in nanophthalmic eyes is encouraging5. The procedure, which might reduce intraocular pressure (IOP), may indeed be an alternative to glaucoma filtration surgery6. Nevertheless, the surgery is associated with a high risk of complications compared to surgery for age related cataracts. Previous studies have found the relationship between anterior chamber depth (ACD) and the IOP6,7, but very little is known about anterior chamber volume (ACV) and anterior chamber angle (ACA). These parameters are as important as anterior chamber depth (ACD). In this study we performed a retrospective analysis of the outcomes of coaxial micro-incision phacoemulsification on anterior chamber profile. We evaluated visual and refractive outcomes and investigated perioperative complications and possible preventative measures among eyes with nanophthalmos.
Currently, the outcome of phacoemulsification and intraocular lens (IOL) implantation in nanophthalmic eyes is encouraging5. The procedure, which might reduce intraocular pressure (IOP), may indeed be an alternative to glaucoma filtration surgery6. Nevertheless, the surgery is associated with a high risk of complications compared to surgery for age related cataracts. Previous studies have found the relationship between anterior chamber depth (ACD) and the IOP6,7, but very little is known about anterior chamber volume (ACV) and anterior chamber angle (ACA). These parameters are as important as anterior chamber depth (ACD). In this study we performed a retrospective analysis of the outcomes of coaxial micro-incision phacoemulsification on anterior chamber profile. We evaluated visual and refractive outcomes and investigated perioperative complications and possible preventative measures among eyes with nanophthalmos.
Materials and methods
Patients and surgical procedures
Our retrospective study involved 89 eyes of 59
patients with nanophthalmos that underwent phacoemulsification
surgery by the same surgeon (Zhaohui Li) from January 2010 to May 2014 at the
PLA General Hospital. All cases were enrolled from
database of medical record management department. This study was approved by the local ethics committee. Our
research adhered to the tenets of the
Declaration of Helsinki. Informed consent was obtained
from all participants. The inclusion criteria
were that the eyes had an AL<21 mm and were
treated with phacoemulsification and IOL implantation. The exclusion criteria included a follow-up period< 3 months; obvious fundus diseases detected
by B-scan echography preoperatively, such as retinal
detachment and choroidal effusion; other anatomic
and structural abnormalities, including chorioretinal
colobomas, retinal dysplasia and persistent hyperplastic
primary vitreous2. In order to investigate the
effect of previous antiglaucomatous surgery on anterior
chamber and complications, cases were divided
into groups of patients who had not undergone previous
surgery (Group A) and those who had a previous
surgical procedure (Group B).
All cases were performed using superficial anesthesia. Viscoelastic was injected as quickly as possible when paracentesis was created to make a "stab" incision. The clear corneal incision was perforated with a 2.2 mm keratome blade. Coaxial micro-incision phacoemulsification was performed by a stop and chop technique using either the Infinity phacoemulsification system (Alcon Laboratories Inc, Irvine, CA, USA) or the Signature phacoemulsification system (Abbott Medical Optics Inc, Andrew Place Santa Ana, CA, USA). The machine settings were as follows: the bottle height was 95 cm; the vacuum was 400-450 mmHg; the power was 60%; the aspiration rate was 38 ml/min. Three types of foldable acrylic IOLs were implanted as appropriate: Akreos Adapt [Bausch&Lomb Inc, Rochester, NY, USA], SN60WF [Alcon Laboratories Inc, Huntington, WV, USA] or AMO ZCB00 [Abbott Medical Optics Inc, Andrew Place Santa Ana, CA, USA]).
All cases were performed using superficial anesthesia. Viscoelastic was injected as quickly as possible when paracentesis was created to make a "stab" incision. The clear corneal incision was perforated with a 2.2 mm keratome blade. Coaxial micro-incision phacoemulsification was performed by a stop and chop technique using either the Infinity phacoemulsification system (Alcon Laboratories Inc, Irvine, CA, USA) or the Signature phacoemulsification system (Abbott Medical Optics Inc, Andrew Place Santa Ana, CA, USA). The machine settings were as follows: the bottle height was 95 cm; the vacuum was 400-450 mmHg; the power was 60%; the aspiration rate was 38 ml/min. Three types of foldable acrylic IOLs were implanted as appropriate: Akreos Adapt [Bausch&Lomb Inc, Rochester, NY, USA], SN60WF [Alcon Laboratories Inc, Huntington, WV, USA] or AMO ZCB00 [Abbott Medical Optics Inc, Andrew Place Santa Ana, CA, USA]).
Measurement of parameters
All biometric parameters were measured within 1
week before and 2 weeks after surgery. ACD, ACV
and ACA were measured using a Pentacam (HR70900, OCULUS Optikgerate GmbH, Wetzlar, Germany). IOP was recorded as the mean of 3 values using a
non-contact tonometer. (NCT [TX-F, Canon Inc, Tokyo, Japan]) and Goldmann applanation tonometer (GAT [Keeler Ltd, Berkshire, UK]). An abnormal
IOP was defined as 21 mmHg or higher (GAT). Two doctors concurred on and graded the corneal diameter
and nuclear hardness by Emery criteria preoperatively. AL
and corneal keratometry were measured
using an IOLMaster (Carl Zeiss Meditec AG, Jena, Germany). The Hoffer Q formula was used for
IOL power calculations. The target refractive diopter, refractive diopter before and 3 months after surgery, and the power of the IOLs were recorded. Best corrected
visual acuity (BCVA) was assessed preoperatively
and 3 months postoperatively, and then converted
into logarithm of the minimum angle of resolution (logMAR) for analysis. BCVA poorer than
logMAR 1.6 were estimated as follows: counting
finger=2.2 logMAR, and hand movement =2.3 logMAR8. All
previous surgical histories and preoperative
complications were identified from the patients' medical records. A positive anterior inflammatory
response (AIR) was in the form of flare and/or cells≥ grade 2 by Hogan's criteria9. If an abnormal IOP
was detected preoperatively, topical brinzolamide (Azopt, s.a.Alcon-Couvreur n.v., Rijksweg, Puurs, Belgium). 1% and timolol maleate (Timoptic, Wujing Inc, WuHan, China) 0.5%, or intravenous mannitol
20% was routinely administrated for better control
of IOP.
Statistical analysis
Statistical analyses were executed using SPSS
software (19.0 version, SPSS Inc). All data were
expressed as the mean and standard deviation. If data
were not normally distributed, medians with 25%
and 75% interquartile ranges. (IQR) were recorded. Differences between groups were analyzed by the
Wilcoxon signed rank test or the Mann-Whitney
test, as appropriate, and the Chi-square test for enumeration
data. Correlations between measurements
were analyzed by Spearman's correlation analysis.
Univariate and multivariate logistic regressions (backward-selection and forward-selection techniques) were
performed to evaluate the odds ratio. Alpha=0.05 for all statistic tests (two sided). A P<0.05 was considered statistically significant.
Results
Patient characteristics
The preoperative characteristics of study patients(mean age was 56.51 years) are summarized in Tables
1 and 2. Female patients accounted for 31 and
male patients accounted for 28 in the study.
Table 1 Demographic data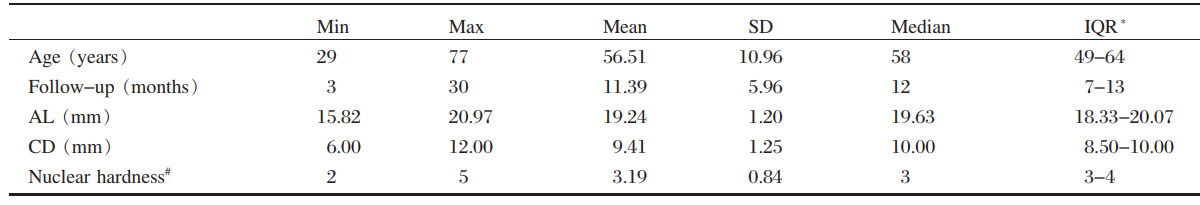

SD= standard deviation; IQR = interquartile ranges; AL =axial length; CD = corneal diameter.
*25% to 75% #According to the Emery nuclear hardness classification
Table 2 Surgical history of all eyes*25% to 75% #According to the Emery nuclear hardness classification
Outcomes of anterior chamber parameters and results of IOP
The biometric parameters measured within 1 week
before and 2 weeks after phacoemulsification surgery
are listed in Table 3. There were significant differences
in ACD, ACV, ACA, before and after surgery(Z=-8.193, -8.193, -7.542, all P< 0.001) and
IOP of NCT and GAT (Z=-6.743, -5.437, all P<0.001). In association with glaucoma, IOP was
compared with anterior chamber parameters. The results
were analyzed by Spearman's rank correlation. Preoperative IOP was negatively correlated with
ACD before and after surgery (P=0.032, r=-0.228; P<0.001, r=-0.475). Postoperative IOP was also
negatively correlated with postoperative ACD (P<0.001, r=-0.377) and ACA (P=0.007, r=-0.285).Parameters such as AL, nuclear hardness, ACD, ACV and ACA between groups A and B before phacoemulsification
surgery were similar (Mann-Whitney
test; Z=-0.380, -0.545, -1.020, -0.690 and 0.277; P=0.704, 0.586, 0.307, 0. 490 and 0.782). The GAT IOP of group B was lower compared to
group A before surgery (Z=-6.007, P<0.001). After
phacoemulsification surgery, group B ACD (Z=-2.676, P=0.007), ACA (Z=-2.685, P=0.007)and GAT IOP (Z=-5.966, P<0.001) were lower compared
to group A.
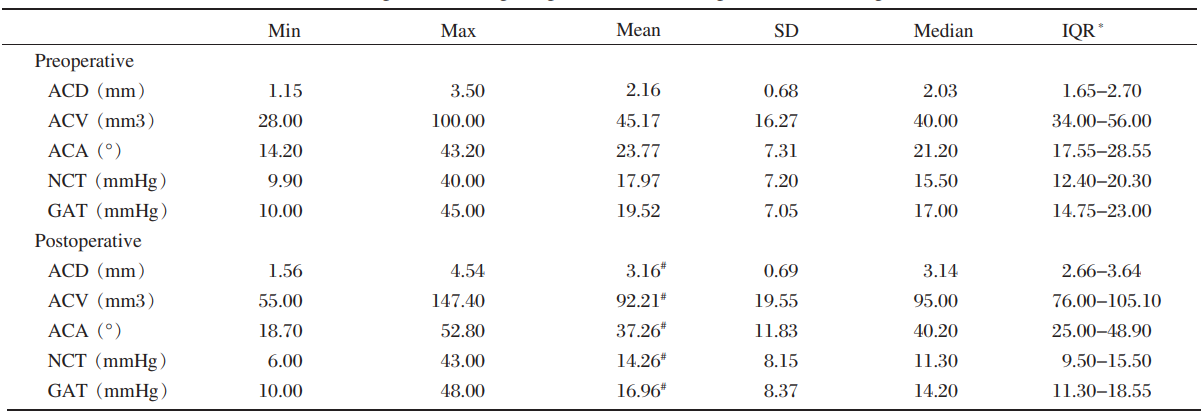
SD=standard deviation; IQR=interquartile ranges; ACD=anterior chamber depth; ACV=anterior chamber volume; ACA=anterior chamber angle; NCT=non-contact tonometer; GAT=Goldmann applanation tonometry.
Table 3 Preoperative and postoperative biometric parameters of all patients

SD=standard deviation; IQR=interquartile ranges; ACD=anterior chamber depth; ACV=anterior chamber volume; ACA=anterior chamber angle; NCT=non-contact tonometer; GAT=Goldmann applanation tonometry.
*25% to 75%
#Wilcoxon signed rank test (all P<0.001)
Visual outcome and refractive results
BCVA and refractive diopter 3 months after
surgery were compared with those before surgery. The median pre- and post-operative logMAR BCVA
were significantly different. (Wilcoxon signed rank
test, Z=-7.999, P<0.001): 1.4 (range 0.8-2.3) and 0.8 (range 0.1-1.4), respectively. The logMAR
BCVA for group B was lower before surgery (Mann-Whitney test, Z=-2.023, P=0.043). The
logMAR BCVA for groups A and B were similar after
surgery (Mann-Whitney test, Z=-0.264, P=0.791). Visual acuity unchanged in 4 eyes of 4 cases (4.49%) and was poorer due to cystoid macular
edema (CME) and uveal effusion in 1 eye of 1 case (1.12%). The degree of hyperopia before surgery
varied from+4.50 D to+20.00 D with a median value
of+9.00 D. After implantation of the IOLs (median
power of 30 D, range 29.00 D to 34.00 D), hyperopia significantly decreased to+3.00 D (range
-0.50 D to 14.00D, Wilcoxon signed rank test, Z=-8.205, P<0.001). The median difference between
target refractive diopter and achieved postoperative
refractive diopter was +0.50 D (range -1.50 D- +4.00
D), and 37 eyes (41.57%) were within±0.50 D.
Perioperative complications
Early stage was defined as less and late stage as
more than 1 month after surgery. Iridoschisis, an intraoperative
complication, was observed in 6 eyes
with glaucoma. Of these, one eye had received
neodymium: YAG laser (YAG) peripheral iridotomy (PI) and 5 eyes had undergone trabeculectomy
and PI. Accordingly, the previous surgeries may
have caused iris trauma and synechia. The most frequent
early postoperative complications were temporary
corneal edema (TCE), AIR, CME and uveal
effusion, while the most frequent late postoperative
complications were CME and uveal effusion. The
incidence of complications were not significantly different
for group A (73.91%) compared to group B (62.12%), (chi-square test, χ2=1.312, P=0.252). Excluding transient TCE and AIR, the incidence of
complications in group A (73.91%) were significantly
higher compared with group B (37.88%, Chisquare
test, χ2=8.887, P=0.003).
The edema and inflammatory response after
surgery were cleared up, and uveal effusion was resolved
in all cases. Treatment of CME was ineffective.
YAG-capsulotomy cleared the posterior capsular
opacification (PCO) in 10 eyes (11.24%) (Table
4). Phacoemulsification surgery did not correct the
elevated IOP in 19 eyes. One eye had normal IOP
and no previous surgical history. Antiglaucomatous
medication was used to manage 18 eyes with high
IOP before surgery, 2 of which developed malignant
glaucoma. Surgical intervention, consisting of YAG
PI, cyclophotocoagulation, trabeculectomy with PI, and YAG-capsulotomy combined with anterior vitrectomy
was performed in 9 eyes (47.37%) which
had high IOP after phacoemulsification surgery. No
other severe complications were detected in the other
10 eyes (52.63%).
The relationship between risk factors and complications was evaluated. The nuclear hardness of patients presenting with inflammatory response significantly differed from that of their counterparts without inflammatory response (Mann-Whitney test, P=0.002). PCO was related to AIR (Spearman's rank correlation, P=0.000, r=0.42). Univariate and multivariate logistic regressions were performed to estimate OR for each variable. Factors were included in the final multivariate model only if significant (P<0.05). The data showed nuclear hardness was related to TCE (P<0.001, odds ratio [OR]=3.42,95% confidence interval [CI]=1.75-6.70); AIR was positively associated with AL (P=0.003, OR=0.44, 95% CI=0.26-0.76) and nuclear hardness(P=0.031, OR=0.44, 95% CI=1.08-4.45); AL (P=0.001, OR=0.28, 95% CI=0.13-0.59), ACA (P=0.020, OR=0.82, 95% CI=0.70-0.97) Mean keratometry (Km) (P=0.001, OR=1.82, 95% CI=1.26-2.64) was related to CME. Uveal effusion was related to AL (P=0.002, OR= 0.01, 95% CI=0.001-0.22).
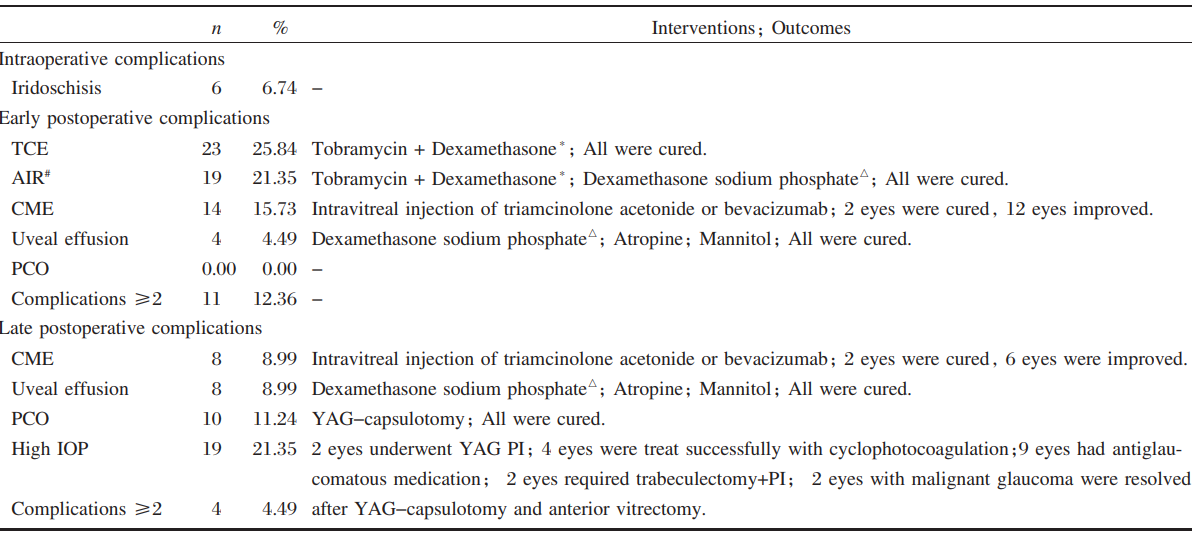 TCE=temporary corneal edema; AIR=anterior inflammatory response; CME=cystoid macular edema; PCO=posterior capsular opacification; YAG=neodymium: YAG laser; High IOP=21 mmHg or higher (Goldmann applanation tonometry); PI=peripheral iridotomy.
TCE=temporary corneal edema; AIR=anterior inflammatory response; CME=cystoid macular edema; PCO=posterior capsular opacification; YAG=neodymium: YAG laser; High IOP=21 mmHg or higher (Goldmann applanation tonometry); PI=peripheral iridotomy.
The relationship between risk factors and complications was evaluated. The nuclear hardness of patients presenting with inflammatory response significantly differed from that of their counterparts without inflammatory response (Mann-Whitney test, P=0.002). PCO was related to AIR (Spearman's rank correlation, P=0.000, r=0.42). Univariate and multivariate logistic regressions were performed to estimate OR for each variable. Factors were included in the final multivariate model only if significant (P<0.05). The data showed nuclear hardness was related to TCE (P<0.001, odds ratio [OR]=3.42,95% confidence interval [CI]=1.75-6.70); AIR was positively associated with AL (P=0.003, OR=0.44, 95% CI=0.26-0.76) and nuclear hardness(P=0.031, OR=0.44, 95% CI=1.08-4.45); AL (P=0.001, OR=0.28, 95% CI=0.13-0.59), ACA (P=0.020, OR=0.82, 95% CI=0.70-0.97) Mean keratometry (Km) (P=0.001, OR=1.82, 95% CI=1.26-2.64) was related to CME. Uveal effusion was related to AL (P=0.002, OR= 0.01, 95% CI=0.001-0.22).
Table 4 Intraoperative, early and late postoperative complications

*Tobramycin+Dexamethasone: topical tobramycin(0.3%)+dexamethasone (0.1%)
#
AIR was documented in the form of flare and/or cells ≥ grade 2 by Hogan's criteria
△Dexamethasone sodium phosphate: intravenous dexamethasone sodium phosphate 5-10 mg daily
Discussion
In our study, there were no complications in the
majority (60.67%) of cases (if TCE and AIR were
excluded), and improvement of BCVA was achieved
in 94.38% of cases. Nonetheless, significant risks are
still associated with phacoemulsification surgery on
nanophthalmic eyes. Compared with a previous study10, we achieved a limited gain in BCVA, with greater
deviation (from 0.1 logMAR to 1.4 logMAR). This
suboptimal visual acuity could be caused by two
factors. Firstly, the majority of patients with nanophthalmos
came from remote rural regions in China, and had poor visual acuity when they were teenagers
or suffered from preexisting ocular diseases such as
refractive amblyopia and foveal underdevelopment. Secondly, most of the eyes included in our study
had glaucoma which could damage the optic nerve
before cataract surgery.
Postoperative BCVA in nanophthalmos was less favorable compared with that in normal eyes. This was also true for the refractive results. The target refraction of ±0.50 D was not reached in 52 eyes (58.43%), most of which remained hyperopic. Several factors may be responsible for this discrepancy, including discrepancies in AL measurement and IOL power calculation. IOL calculations could be inaccurate, with the Holladay Ⅱ formula typically being preferred although it is not readily available11-12. The Hoffer Q formula which performed as well as the HolladayⅡ formula for small eyes13 is readily available and therefore was our method of choice. Although Gayton et al14 demonstrated 2 IOLs , in a piggyback manner, can be successfully implanted, we considered that the zonules in those small eyes may be too weak to support insertion of 2 IOLs. Oshika et al15 modified the technique by implanting 1 IOL in the capsular bag and another IOL in the sulcus. We determined that insufficient space was available for 2 IOLs, and more importantly, that piggyback IOLs would produce marked hyperopic drift16. Based on all of these factors, we used a single highpower IOL instead of piggyback IOLs.
Phacoemulsification lens surgery of nanophthalmic eyes is associated with a high risk of perioperative complications, such as corneal decompensation, severe iritis, explosive choroidal hemorrhage, uveal effusion, retinal detachment, malignant glaucoma and CME17. In our cases, TCE, AIR, CME and uveal effusion accounted for the majority of early postoperative complications, while CME, uveal effusion glaucoma and PCO accounted for the majority of late postoperative complications. Nuclear hardness was a major risk factor for TCE and AIR. Once CME occurred, it was difficult to treat.In our study 2 eyes of 2 patients developed malignant glaucoma, presumably because of capsular block and aqueous misdirection and required YAG-capsulotomy combined with anterior vitrectomy to manage the IOP. Both patients had intractable IOP elevation (1 eye had cyclophotocoagulation) before surgery. We deemed that additional surgical interventions were often required as soon as complications were detected, emphasizing the necessity for careful follow-up.
In our study, there were no severe complications. One of the reasons for this may be that the percentage of high-risk eyes (approximately <18 mm18) was low (17.98%). More importantly, we believed the reduction of severe complications was closely related to surgical technique, and the surgery should always be handled by an experienced surgeon. To protect the corneal endothelium, we injected low density vis coelastic (VISCOAT, Alcon Laboratories Inc, Texas, USA) before capsulorrhexis. The coaxial micro-incision technique with a 2.2 mm corneal incision reduced the trauma around the corneal incision site. A stop and chop technique was performed to divide the lens as small as possible (approximately 6-8 pieces). We created paracentesis to make a “stab” incision for the first step, and made the viscoelastic advancing towards the paracentesis to gradually replace the aqueous humor. This may help reduce the aqueous outflow and naturally maintain ACD. We surmised that a 95 cm bottle height may be suitable, as it could maintain normal ACD without causing ocular hypertension or ischemia during surgery. We preferred topical anesthesia to retrobulbar anesthesia, because it reduced the vitreous pressure. Preoperative administration of mannitol may be important in preventing unexpected complications19. As expected, we noticed that previous surgical intervention may be helpful for postoperative ACD and IOP, and may protect against the development of complications. The risk for complications in nanophthalmos was greater with reducing AL, ACD, ACV or ACA and with higher nuclear hardness or Km.
Our study represented a fairly comprehensive case series. A previous study indicated that when the AL cut off to define nanophthalmos was <20.9 mm, 0.1 mm less than the 21 mm AL we used to defined nanophthalmos, the sample size of the present study would reduce the by nearly 25%20. By and large, the outcomes that we report were gratifying. Our experience may be useful to surgeons and patients at the time of perioperative preparation and preoperative counseling. The present study was related to the techniques and skills of an individual surgeon, potentially limiting the generalization of our results. IOLs with a power greater than 34 D were not readily available. This could leave nanophthalmos eyes with a higher postoperative refractive diopter receiving less powerful IOLs. Further studies may be more valuable if we included even short eyes. The decision to use ocular hypotensive agents was based on clinical judgment, and patients were not randomized into groups. We only considered the risk factors correlated with the surgery when we studied the incidence of PCO, ignoring the influence of previous holopathy such as diabetes and hypertension. Therefore, our results may be somewhat biased. Future largescale multicenter randomized controlled clinical trials are recommended to generalize our outcomes.
Postoperative BCVA in nanophthalmos was less favorable compared with that in normal eyes. This was also true for the refractive results. The target refraction of ±0.50 D was not reached in 52 eyes (58.43%), most of which remained hyperopic. Several factors may be responsible for this discrepancy, including discrepancies in AL measurement and IOL power calculation. IOL calculations could be inaccurate, with the Holladay Ⅱ formula typically being preferred although it is not readily available11-12. The Hoffer Q formula which performed as well as the HolladayⅡ formula for small eyes13 is readily available and therefore was our method of choice. Although Gayton et al14 demonstrated 2 IOLs , in a piggyback manner, can be successfully implanted, we considered that the zonules in those small eyes may be too weak to support insertion of 2 IOLs. Oshika et al15 modified the technique by implanting 1 IOL in the capsular bag and another IOL in the sulcus. We determined that insufficient space was available for 2 IOLs, and more importantly, that piggyback IOLs would produce marked hyperopic drift16. Based on all of these factors, we used a single highpower IOL instead of piggyback IOLs.
Phacoemulsification lens surgery of nanophthalmic eyes is associated with a high risk of perioperative complications, such as corneal decompensation, severe iritis, explosive choroidal hemorrhage, uveal effusion, retinal detachment, malignant glaucoma and CME17. In our cases, TCE, AIR, CME and uveal effusion accounted for the majority of early postoperative complications, while CME, uveal effusion glaucoma and PCO accounted for the majority of late postoperative complications. Nuclear hardness was a major risk factor for TCE and AIR. Once CME occurred, it was difficult to treat.In our study 2 eyes of 2 patients developed malignant glaucoma, presumably because of capsular block and aqueous misdirection and required YAG-capsulotomy combined with anterior vitrectomy to manage the IOP. Both patients had intractable IOP elevation (1 eye had cyclophotocoagulation) before surgery. We deemed that additional surgical interventions were often required as soon as complications were detected, emphasizing the necessity for careful follow-up.
In our study, there were no severe complications. One of the reasons for this may be that the percentage of high-risk eyes (approximately <18 mm18) was low (17.98%). More importantly, we believed the reduction of severe complications was closely related to surgical technique, and the surgery should always be handled by an experienced surgeon. To protect the corneal endothelium, we injected low density vis coelastic (VISCOAT, Alcon Laboratories Inc, Texas, USA) before capsulorrhexis. The coaxial micro-incision technique with a 2.2 mm corneal incision reduced the trauma around the corneal incision site. A stop and chop technique was performed to divide the lens as small as possible (approximately 6-8 pieces). We created paracentesis to make a “stab” incision for the first step, and made the viscoelastic advancing towards the paracentesis to gradually replace the aqueous humor. This may help reduce the aqueous outflow and naturally maintain ACD. We surmised that a 95 cm bottle height may be suitable, as it could maintain normal ACD without causing ocular hypertension or ischemia during surgery. We preferred topical anesthesia to retrobulbar anesthesia, because it reduced the vitreous pressure. Preoperative administration of mannitol may be important in preventing unexpected complications19. As expected, we noticed that previous surgical intervention may be helpful for postoperative ACD and IOP, and may protect against the development of complications. The risk for complications in nanophthalmos was greater with reducing AL, ACD, ACV or ACA and with higher nuclear hardness or Km.
Our study represented a fairly comprehensive case series. A previous study indicated that when the AL cut off to define nanophthalmos was <20.9 mm, 0.1 mm less than the 21 mm AL we used to defined nanophthalmos, the sample size of the present study would reduce the by nearly 25%20. By and large, the outcomes that we report were gratifying. Our experience may be useful to surgeons and patients at the time of perioperative preparation and preoperative counseling. The present study was related to the techniques and skills of an individual surgeon, potentially limiting the generalization of our results. IOLs with a power greater than 34 D were not readily available. This could leave nanophthalmos eyes with a higher postoperative refractive diopter receiving less powerful IOLs. Further studies may be more valuable if we included even short eyes. The decision to use ocular hypotensive agents was based on clinical judgment, and patients were not randomized into groups. We only considered the risk factors correlated with the surgery when we studied the incidence of PCO, ignoring the influence of previous holopathy such as diabetes and hypertension. Therefore, our results may be somewhat biased. Future largescale multicenter randomized controlled clinical trials are recommended to generalize our outcomes.
Conclusion
With reasonable preoperative management, prudent
selections of the lens, rigorous surgical technique, and unerring cognition of the potential complications, coaxial
micro-incision phacoemulsification
surgery can be favorably performed in nanophthalmos
with good results. Nonetheless, the surgery
in nanophthalmos is still challenging, primarily due
to the high incidence of complications.
基金
暂无基金信息
参考文献
1、Wu W, Dawson DG, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications[J]. J Cataract Refract Surg, 2004, 30(3): 584-590.
2、Wladis EJ, Gewirtz MB, Guo S. Cataract surgery in the small adult eye[J]. Surv Ophthalmol, 2006, 51(2): 153-161.
3、Singh OS, Simmons RJ, Brockhurst RJ, et al. Nanophthalmos:a perspective on identification and therapy [J].Ophthalmology, 1982, 89(9): 1006-1012.
4、Auffarth GU, Blum M, Faller U, et al. Relative anterior microphthalmos: morphometric analysis and its implications for cataract surgery[J].Ophthalmology, 2000,107(8):1555-1560.
5、Day AC, MacLaren RE, Bunce C, et al. Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos[J].J Cataract Refract Surg,2013,39(1):87-96.
6、Seki M, Fukuchi T, Ueda J, et al. Nanophthalmos:quantitative analysis of anterior chamber angle configuration before and after cataract surgery [J].Br J Ophthalmol, 2012,96(8):1108-1116.
7、Sharan S, Grigg JR,Higgins RA.Nanophthalmos: ultrasound biomicroscopy and Pentacam assessment of angle structures before and after cataract surgery[J].J Cataract Refract Surg, 2006, 32(6): 1052-1055.
8、Riusala A, Sarna S, Immonen I.Visual function index(VF-14) in exudative age-related macular degeneration of long duration[J].Am J Ophthalmol,2003,13(2): 206-212.
9、Hogan MJ, Kimura SJ, Thygeson P.Signs and symptoms of uveitis.I.Anterior uveitis[J]. Am J Ophthalmol,1959, 47(5 Pt 2):155-170.
10、Ventura MC, Sampaio VV, Ventura BV, et al. Congenital cataract surgery with intraocular lens implantation in microphthalmic eyes: visual outcomes and complications
[J]. Arq Bras Oftalmol, 2013, 76(4): 240-243.
11、Day AC, Foster PJ, Stevens JD. Accuracy of intraocular lens power calculations in eyes with axial length <22. 00 mm [J]. Clin Experiment Ophthalmol, 2012, 40 (9):855-862.
12、Hoffer KJ. Clinical results using the Holladay 2 intraocular lens power formula [J]. J Cataract Refract Surg, 2000, 26 (8) : 1233-1237.
13、Jung KI, Yang JW, Lee YC, et al. Gayton JL, Sanders VN. Implanting two posterior chamber intraocular lenses in a case of microphthalmos[J]. J Cataract Refract Surg,
1993, 19(6): 776-777.
14、Oshika T, Imamura A, Amano S, et al. Piggyback foldable intraocular lens implantation in patients with microphthalmos[J]. J Cataract Refract Surg, 2001, 27(6):841-844.
15、Fenzl RE, Gills JP, Cherchio M. Refractive and visual outcome of hyperopic cataract cases operated on before and after implementation of the Holladay II formula [J].
Ophthalmology, 1998, 105(9): 1759-1764.
16、Yuzbasioglu E, Artunay O, Agachan A, et al. Phacoemulsification in patients with nanophthalmos[J]. Can J Ophthalmol, 2009, 44(5): 534-539.
17、Vijaya L, Rewri P, George R, et al. Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos[J]. Am J Ophthalmol, 2012, 154(5): 913-914;author reply 914.
18、Pahor D, Gracner T, Gracner B. Cataract surgery in nanophthalmic eyes[J]. Klin Monbl Augenheilkd, 2012,229(11): 1113-1117.
19、Nerone LD. Cataract surgery and microphthalmic eyes[J].J Cataract Refract Surg, 2013, 39(5): 817.
相关文章
蔡晨希;Jin-Li Cui;Qiang Wang;Tao Li;Bing-Qian Liu;Zhen-Qiang Lin;Xiao-Mei Xiong;Ze-Hao Liu;Ying Lin,Visual prognosis of vitrectomy for polypoidal choroidal vasculopathy with breakthrough vitreous hemorrhage Zhenggen Wu;Chukai Huang;Ce Zheng; Yuqiang Huang;Wanqi Zhang; Di Ma,The safety and effi cacy of modifi ed minimally invasive trabeculectomy for the treatment of primary chronic angle-closure glaucomaHuijing Ye;Xiufen Lian;Rongxin Chen;Yanling Zhu;Jingxia Huang;Hongbin Chen;Ling Xie;Wenjun Guo;Huasheng Yang,Folded technique of self-adherent wrap improves clinical outcomes for wounds after orbital tumour extirpation: a single-center, prospective randomized controlled trial